Ambisome IV Vial
- Introduction to Ambisome IV Vial
- Composition and Formulation
- Uses of Ambisome IV Vial
- Amphotericin b mechanism of action
- Amphotericin b dose and Administration Guidelines
- Amphotericin b side effects
- Contraindications and Warnings
- Important Precautions
- Amphotericin b administration protocol
- Amphotericin b interactions
- Storage and Handling of Ambisome IV Vial
- Overdosage and Emergency Management
- Handling Precautions for Healthcare Providers
Introduction to Ambisome IV Vial
Overview of Ambisome
Ambisome represents a pharmaceutical breakthrough created to effectively address fungal infections. It is developed as a version of amphotericin B, with a treatment strategy aimed at reducing overall toxicity in the body.
Purpose and Significance in Medical Treatment
Ambisome plays a role in treating fungal infections that are especially concerning for people with weakened immune systems. Its effectiveness in providing properties while safeguarding essential organ functions highlights its significance in contemporary healthcare.
FDA Approval and Classification
The FDA has approved Ambisome as a cutting-edge antifungal treatment option. It falls into the polyene antifungal group known for its ability to interfere with the integrity of fungal cells.
Summary of Its Primary and Off-Label Uses
Ambisome is commonly utilized for treating infections and visceral leishmaniasis; however, it is also being investigated for alternative uses, such as managing febrile neutropenia and cryptococcal meningitis in individuals with HIV/AIDS.
Composition and Formulation
Active Ingredient: Liposomal Amphotericin B
The main active component in the medication is amphotericin B, which is enclosed in liposomes to improve its absorption in the body and minimize kidney damage.
Excipient Components and Their Roles
- Cholesterol helps to maintain the stability of the structure.
- Phospholipids help in the encapsulation and delivery of drugs.
- Sodium succinate helps with adjusting pH levels to ensure stability.
Mechanism of Liposomal Delivery for Enhanced Efficacy
The liposomal formulation allows amphotericin B to evade rapid systemic clearance, ensuring targeted fungal eradication while sparing human cells.
Abelcet vs ambisome
Abelcet has a price to AmBlsome. It leads to more kidney-related issues and side effects during the infusion process.
Itraconazole vs ambisome
Itraconazole and amphotericin B are medications used to combat fungal infections, commonly known as Ambisome, in the field. Itraconazole is effective against yeast infections affecting the mouth and throat, as well as fungal conditions such as aspergillosis and blastomycosis. Ambisome is prescribed for infections that do not respond well to other forms of amphotericin treatment or when the patient does not tolerate them well.
Amphotericin b deoxycholate
A drug from the polyene class that's effective against fungi and is currently the treatment for most fungal infections in the central nervous system (CNS). Polyenes attach to ergosterol in the wall of cells, disrupting the cell structure by changing its permeability and ultimately leading to the death of the fungus.
Uses of Ambisome IV Vial
Primary Uses
- Treatment of Systemic Fungal Infections: Effective against invasive candidiasis and aspergillosis.
- Management of Visceral Leishmaniasis: A cornerstone therapy in endemic regions.
Off-Label Uses
- Empirical Therapy for Febrile Neutropenia: Aids in addressing potential fungal pathogens.
- Cryptococcal Meningitis in HIV/AIDS Patients: A life-saving adjunct in antifungal therapy.
- Treatment of Invasive Aspergillosis: Explored when conventional therapies fail.
- Potential Use in Severe Viral Infections: Emerging studies highlight its antiviral synergy.
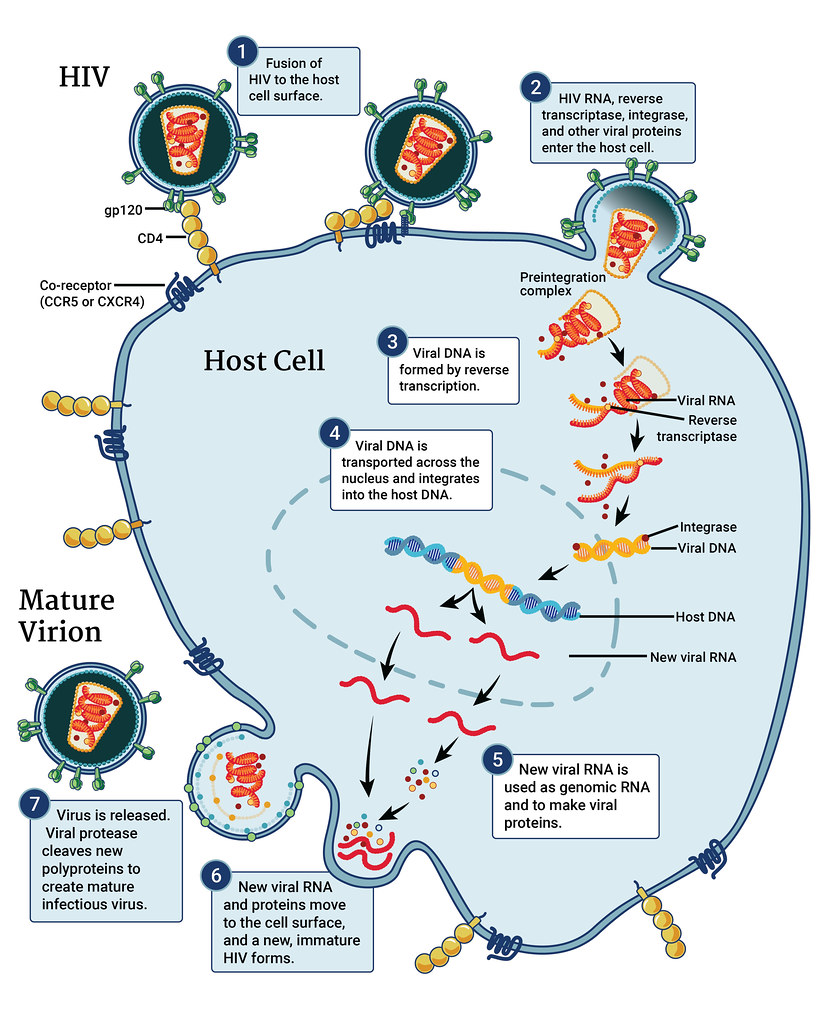
Amphotericin b mechanism of action
Mechanism of Action: Disruption of Fungal Cell Membranes
Ambisome attaches to ergosterol in the membrane. Forms pores that result in the demise of the cell.
Role of Liposomal Formulation in Reducing Toxicity
Enclosing amphotericin B in liposomes helps reduce the effects on the kidneys by targeting the infected tissues.
Pharmacokinetics and Absorption
- Prolonged systemic circulation ensures sustained antifungal action.
- Minimal renal uptake preserves kidney function.
Amphotericin b dose and Administration Guidelines
Standard Dosing for Primary Indications
- Fungal infections: 3-5 mg/kg daily.
- Visceral leishmaniasis: 3 mg/kg on specific schedules.
Dose Adjustments for Renal or Hepatic Impairment
Titrate doses based on creatinine clearance and liver enzyme levels to avoid toxicity.
Administration Techniques: IV Infusion Protocols
- Infuse over 30-60 minutes.
- Use a dedicated IV line to prevent interactions.
Monitoring During Administration
Remember to monitor signs such as heart rate and blood pressure and check levels and kidney function before and after administering the infusion.
Amphotericin b side effects
Common Side Effects
- Transient fever and chills.
- Electrolyte imbalances, particularly hypokalemia.
Serious Side Effects
- Anaphylactic reactions requiring immediate intervention.
- Renal impairment demanding close monitoring.
- Infusion-related hypotension and tachycardia.
Contraindications and Warnings
Absolute Contraindications
- Hypersensitivity to amphotericin B or its components.
Situational Contraindications
- Avoid in patients with severe renal impairment unless no alternatives exist.
Critical Warnings
- Monitor for signs of hepatotoxicity.
- Prepare for emergency management of hypersensitivity reactions.
Important Precautions
Pre-Administration Screening for Allergies and Organ Function
Conduct thorough pre-treatment assessments, including allergy tests and renal function evaluation.
Importance of Monitoring Kidney Function
Frequent creatinine and electrolyte monitoring is essential to detect early signs of toxicity.
Mitigation of Infusion-Related Reactions
- Premedicate with antihistamines or corticosteroids when necessary.
- Slow infusion rates to reduce adverse effects.
Amphotericin b toxicity
The main immediate side effects of Amphotericin B deoxycholate are nausea and vomiting, along with chills and fever, followed by low blood pressure and reduced oxygen levels in the body's tissues (hypoxia). Its common term negative impact is kidney damage caused by Amphotericin B, likely through multiple different processes.
Amphotericin b electrolyte abnormalities
Some common electrolyte issues involve a decrease in potassium and magnesium due to the cell membranes becoming more permeable over time. This results in trouble retaining hydrogen ions in the collecting duct, which can cause tubular acidosis (DRTA).
Amphotericin b administration protocol
Elderly Patients
Older patients commonly pose difficulties because of the changes associated with aging. The dosage of Ambisome, for this demographic needs modifications primarily addressing kidney function. Adjustments to the dosage might be needed for individuals with filtration rates. It is crucial to monitor kidney function and levels of electrolytes to prevent any effects, from occurring.
Pregnant Women and Nursing Mothers
The safety profile of Ambisome during pregnancy and lactation is a subject of ongoing research. Current evidence suggests that its liposomal formulation reduces systemic toxicity, making it a safer option compared to conventional amphotericin B. However, the use of Ambisome during pregnancy should be guided by a careful risk-benefit analysis, especially in cases of life-threatening fungal infections. For nursing mothers, the transfer of amphotericin B through breast milk appears minimal, but caution is advised.

Pediatric Patients
Pediatric administration requires careful adherence to weight-based dosing guidelines.
- For children and neonates, doses typically range from 1-5 mg/kg depending on the indication.
- Preterm infants may exhibit altered pharmacokinetics, necessitating individualized dosing and extended monitoring.
Amphotericin b interactions
Common Drug Interactions
The interaction profile of Ambisome demands vigilance to avoid adverse effects. -
Concurrent Nephrotoxic Agents: Combining Ambisome with drugs such as aminoglycosides or cyclosporine may exacerbate nephrotoxicity.
- Medications Affecting Electrolyte Balance: Co-administration with diuretics can worsen hypokalemia, necessitating regular electrolyte monitoring.
Effects of Antifungal Combination Therapy
While combining antifungal agents like azoles or echinocandins can enhance efficacy, it may also amplify toxicity risks. Dose adjustments are often necessary.
Interactions with Immunosuppressive Drugs
For individuals with weakened systems, receiving Ambisome treatment may potentially lead to kidney problems when used alongside medications such as tacrolimus or corticosteroids; hence, it is advisable to monitor kidney function during combined therapy.
Storage and Handling of Ambisome IV Vial
Recommended Storage Conditions
- Ambisome vials should be stored at temperatures between 2-8°C.
- Exposure to direct sunlight must be avoided to maintain stability.
Temperature and Light Exposure
Improper storage conditions can lead to degradation of the liposomal formulation, compromising its efficacy. Protect reconstituted solutions from excessive heat and light.
Shelf Life and Stability After Reconstitution
After being reconstituted Ambisome remains stable for a period of 24 hours when kept at a temperature range of 2 to 4 degrees Celsius.
Make sure to dispose of any portions after the recommended time to keep everything.
Proper Handling Precautions to Ensure Sterility
- Use aseptic techniques during preparation and administration.
- Employ sterile water for injection to reconstitute the vial.
Overdosage and Emergency Management
Symptoms of Overdose
Ambisome overdose is a rare but serious event that can manifest with:
- Severe renal impairment, indicated by elevated creatinine levels.
- Respiratory distress, particularly in vulnerable patients.
Immediate Steps in Overdose Management
- Discontinue Ambisome infusion immediately.
- Initiate supportive care to stabilize the patient's vitals.
Supportive Care and Symptomatic Treatment
Ensure the balance of electrolytes by administering supplements. In instances administer assistance as required.
Hemodialysis or Other Detoxification Methods
Amphotericin B is not easily eliminated through hemodialysis treatment. Ensuring hemodynamics remains crucial in managing cases of overdose.
Handling Precautions for Healthcare Providers
Preparation and Reconstitution Best Practices
- Reconstitute Ambisome vials in a controlled environment, using sterile equipment to prevent contamination.
- Avoid shaking the vial vigorously to maintain the integrity of the liposomal formulation.
Protective Measures for Handling Cytotoxic Agents
- Wear gloves and protective eyewear during preparation and administration.
- Dispose of unused product and materials according to local cytotoxic waste guidelines.
Avoidance of Contamination During IV Administration
- Use a dedicated IV line for Ambisome to prevent cross-contamination.
- Employ in-line filters to remove particulate matter during infusion.
Ambisome IV Vial FAQ
- Why amphotericin b causes hypokalemia?
- Why is amphotericin B given in combination with flucytosine?
- Why amphotericin b should be protected from light?
- Where is amphotericin b metabolized?
- When to stop amphotericin b?
- When is amphotericin b used?
- When should amphotericin b be prescribed?
- What is amphotericin b deoxycholate?
- What is amphotericin b mainly used for?
- What does amphotericin b do?
- What does amphotericin b treat?
- What is amphotericin b used for?
- How does amphotericin b cause hypomagnesemia?
- How is amphotericin b administered?
- How does amphotericin b work?
- How amphotericin b causes nephrotoxicity?
- How amphotericin b causes hypokalemia?
- How amphotericin b works?
- Can amphotericin b be mixed with normal saline?
- Can amphotericin b cause deafness?
- Can amphotericin b be given with rbc blood transfusion?
- Can amphotericin be given orally?
- Why amphotericin b causes hypokalemia?
- Why is amphotericin B given in combination with flucytosine?
- Why amphotericin b should be protected from light?
- Where is amphotericin b metabolized?
- When to stop amphotericin b?
- When is amphotericin b used?
- When should amphotericin b be prescribed?
- What is amphotericin b deoxycholate?
- What is amphotericin b mainly used for?
- What does amphotericin b do?
- What does amphotericin b treat?
- What is amphotericin b used for?
- How does amphotericin b cause hypomagnesemia?
- How is amphotericin b administered?
- How does amphotericin b work?
- How amphotericin b causes nephrotoxicity?
- How amphotericin b causes hypokalemia?
- How amphotericin b works?
- Can amphotericin b be mixed with normal saline?
- Can amphotericin b cause deafness?
- Can amphotericin b be given with rbc blood transfusion?
- Can amphotericin be given orally?
Why amphotericin b causes hypokalemia?
Amphotericin B leads to potassium levels by attaching to cells in the tubular collecting ducts and forming channels that enable potassium leakage outwards.
Why is amphotericin B given in combination with flucytosine?
To treat infections effectively, doctors often prescribe a combination of Amphotericin B and flucytosine. This approach is preferred as it is more potent than using either drug on its own. It also helps reduce the risk of developing drug resistance.
Why amphotericin b should be protected from light?
Amphotericin B (AMB) should be shielded from light as it may break down when exposed to heat and low acidity.
Where is amphotericin b metabolized?
Most of the amphotericin B deoxycholate is eliminated from the body without being changed through urine and feces in humans, indicating that humans do not extensively break down amphotericin B in their bodies.
When to stop amphotericin b?
It might be important to keep taking this medicine for weeks to months to completely cure specific infections, as stopping the medicine too soon could lead to the infection coming back.
When is amphotericin b used?
Amphotericin B is mainly used to treat infections that can worsen and pose a risk to life.
When should amphotericin b be prescribed?
It is intended to treat infections that may pose a risk to life.
What is amphotericin b deoxycholate?
Amphotericin B deoxycholate is classified as a type of medication known as polyene.
What is amphotericin b mainly used for?
Amphotericin B is a medication authorized by the FDA for medical use to treat infections, such as histoplasmosis and cryptococcosis.
What does amphotericin b do?
Amphotericin B injection is prescribed for infections that could be life-threatening.
What does amphotericin b treat?
Amphotericin B is mainly used to treat advancing infections. It could be life-threatening.
What is amphotericin b used for?
The management of infections, like histoplasmosis and cryptococcosis, is essential.
How does amphotericin b cause hypomagnesemia?
Amphotericin B, also known as (AmB), may lead to low magnesium levels in the body by harming the kidneys and resulting in magnesium loss through urine.
How is amphotericin b administered?
Amphotericin B injection is typically administered intravenously over a span of 2 to 6 hours, with an injection process involved.
How does amphotericin b work?
The medication functions by attaching to sterols (ergosterol) in the cell membrane of fungi.
How amphotericin b causes nephrotoxicity?
Amphotericin B attaches to sterols found in cell membranes and forms pores that affect the integrity of the membrane and enhance its permeability.
How amphotericin b causes hypokalemia?
When Amphotericin B attaches to cells in the kidneys' collecting duct, it creates openings that allow potassium to leak out, causing hypokalemia.
How amphotericin b works?
The medication works by attaching to sterols (ergosterol found in the cell membrane of fungi). This leads to the formation of a transmembrane passage. Alters the membranes permeability allowing the release of cell components.
Can amphotericin b be mixed with normal saline?
Amphotericin B should not be mixed with sodium chloride as they are substances. Hence it is crucial to avoid infusing this medication with saline solutions containing sodium chloride.
Can amphotericin b cause deafness?
Amphotericin B may lead to alterations in hearing patterns to some extent without reaching significance.
Can amphotericin b be given with rbc blood transfusion?
Giving Amphotericin B at the time of platelet transfusions can have an effect and should be avoided.
Can amphotericin be given orally?
The oral method allows for the administration of amphotericin B, with side effects, but it has limitations in enterocyte permeability.
Why amphotericin b causes hypokalemia?
Amphotericin B induces hypokalemia by attaching to cells in the collecting ducts and forming pores that result in potassium leakage.
Why is amphotericin B given in combination with flucytosine?
Amphotericin B is typically administered alongside flucytosine to combat infections. Their combined effectiveness surpasses that of either drug used individually and aids in reducing the development of drug resistance.
Why amphotericin b should be protected from light?
It is important to shield Amphotericin B (AMB) from light, as exposure to light can lead to its degradation under conditions of exposure and heat, along with pH levels.
Where is amphotericin b metabolized?
Humans tend to eliminate amphotericin B deoxycholate through urine and feces without the drug's metabolism occurring within their bodies.
When to stop amphotericin b?
To effectively address infections it might be required to take this medication for a weeks to several months without interruption; otherwise the infection could reoccur if the medication is discontinued prematurely.
When is amphotericin b used?
Amphotericin B is mainly used to treat infections that are getting worse and could be life-threatening.
When should amphotericin b be prescribed?
It is recommended that it be utilized to address infections that could pose a risk to one's life.
What is amphotericin b deoxycholate?
Amphotericin B deoxycholate is part of the group of medications known as polyenes.
What is amphotericin b mainly used for?
Amphotericin B is a medication used to treat infections, such as histoplasmosis and cryptococcosis, that has been sanctioned by the U.S. Food and Drug Administration (FDA).
What does amphotericin b do?
Amphotericin B injection is prescribed for the treatment of infections that could be life-threatening.
What does amphotericin b treat?
Amphotericin B is mainly used to treat infections that can worsen and pose a threat to one's life.
What is amphotericin b used for?
The management of kinds of infections, like histoplasmosis and cryptococcosis.
How does amphotericin b cause hypomagnesemia?
Amphotericin B, also known as Amtovercin, may lead to magnesium levels by harming the kidneys and inducing magnesium loss through the kidneys.
How is amphotericin b administered?
Amphotericin B injection is typically administered through an infusion lasting between 2 to 6 hours daily.
How does amphotericin b work?
The medication works by attaching to sterols ( ergosterol ) in the cell membrane of fungi that are vulnerable to its effects.
How amphotericin b causes nephrotoxicity?
Amphotericin B attaches, to the sterols found in cell membranes and forms pores that membrane structure and enhance membrane permeability.
How amphotericin b causes hypokalemia?
When Amphotericin B attaches to the cells in the collecting duct, it creates pores that release potassium, leading to hypokalemia.
How amphotericin b works?
The medication works by attaching to sterols (ergosterol) found in the cell membrane of fungi, which creates a transmembrane channel that alters membrane permeability and allows components to leak.
Can amphotericin b be mixed with normal saline?
Amphomycin B should not be mixed with sodium chloride as it can cause compatibility issues. Hence, it's crucial to avoid infusing this medication simultaneously with saline solutions.
Can amphotericin b cause deafness?
Amphotericin B may lead to an alteration in hearing patterns. The difference is not considered statistically significant.
Can amphotericin b be given with rbc blood transfusion?
Amphotericin B may have effects on platelet transfusions. It should not be administered concurrently with them.
Can amphotericin be given orally?
The oral method allows for the delivery of amphotericin B while significantly reducing side effects. However, the permeation through enterocytes still poses a limitation.