Aricept Evess
- Introduction
- Donepezil hydrochloride uses
- How Aricept Evess Works
- Composition of Aricept Evess
- Dosage and Administration
- Storage Guidelines for Aricept Evess
- Donepezil hydrochloride side effects
- Drug Interactions
- Warnings and Contraindications
- Precautions for Administration
- Administration to Elderly Patients
- Administration to Pregnant Women and Nursing Mothers
- Administration to Children
- Overdosage and Management
- Handling Precautions for Aricept Evess
Introduction
Overview of Aricept Evess
Aricept Evess, a revolutionary formulation of donepezil hydrochloride, is a widely used medication for managing cognitive impairments. Its development marked a pivotal advancement in neurodegenerative disorder therapy, particularly for Alzheimer's disease.
Brief History and Development of Aricept Evess
The introduction of Aricept Evess stems from rigorous research to enhance patient adherence and therapeutic outcomes. Its rapid-dissolving tablet technology was designed to address swallowing difficulties, a common issue in Alzheimer's patients.
Primary Therapeutic Use in Alzheimerâs Disease
Aricept Evess is primarily prescribed for Alzheimer's disease. It mitigates symptoms by preserving cognitive function, aiding in daily living activities, and improving quality of life.
Significance in Managing Cognitive Decline
Cognitive decline severely impacts independence and functionality. Aricept Evess plays a crucial role in slowing progression, offering hope for patients and caregivers alike.
Donepezil hydrochloride uses
Approved Uses for Alzheimer's Disease Treatment
Aricept Evess is FDA-approved for treating mild, moderate, and severe Alzheimer's disease. Its effectiveness lies in enhancing communication between brain cells.
Role in Mild, Moderate, and Severe Dementia
- Mild: Improves memory and focus.
- Moderate: Assists with decision-making and routine activities.
- Severe: Maintains basic functions and delays advanced symptoms.
Off-Label Uses of Aricept Evess
Parkinsonâs Disease Dementia
It is utilized to address memory challenges in individuals with Parkinson's disease and demonstrates effectiveness in managing these issues.
Lewy Body Dementia
Improves symptoms like hallucinations and cognitive instability associated with this form of dementia.
Vascular Dementia
Improves functions impacted by decreased blood circulation to the brain.
Cognitive Impairment in Traumatic Brain Injury
Support is growing for its effectiveness in rehabilitation after an injury.
Emerging Therapeutic Applications and Ongoing Research
Research is being conducted to investigate how it could be beneficial, for individuals with autism spectrum disorders and those experiencing memory decline due, to aging.
How Aricept Evess Works
Mechanism of Action as an Acetylcholinesterase Inhibitor
Aricept Evess works by blocking acetylcholinesterase, an enzyme that breaks down acetylcholine in the brain. This helps boost levels of neurotransmitters that are important for memory and learning processes.
Impact on Neurotransmitter Levels in the Brain
The sustained presence of acetylcholine facilitates synaptic transmission, promoting better neuronal communication.
Cognitive Benefits and Limitations
- Benefits: Slows cognitive decline, improves concentration, and boosts awareness.
- Limitations: Does not halt disease progression; effects vary between individuals.
Onset of Action and Duration of Effect
Noticeable improvements may appear within weeks. The effects persist with consistent administration, though individual response times vary.
Composition of Aricept Evess
Active Ingredient: Donepezil Hydrochloride
The primary ingredient in Donepezil hydrochloride, is crucial for its impact on function.
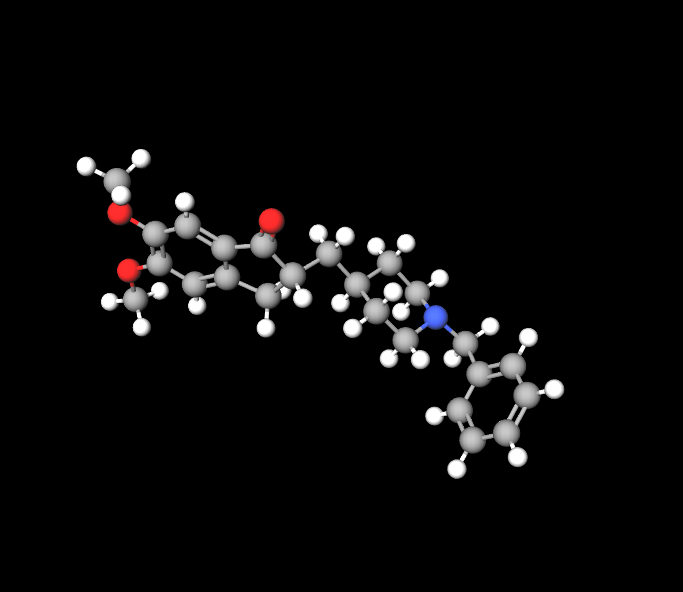
Excipient Profile
It contains inactive ingredients like lactose monohydrate and magnesium stearate to aid in stability and tablet formulation.
Donepezil hydrochloride dosage forms
Available in standard and orally disintegrating tablets, with doses ranging from 5 mg to 23 mg for flexible therapy options.
Dosage and Administration
Recommended Dosages for Different Stages of Alzheimerâs
- 5 mg daily for initial treatment.
- Increased to 10 mg based on tolerance.
- 23 mg for advanced stages under medical guidance.
Best time to take donepezil hydrochloride
It's an idea to take your donepezil before going to bed since it might make you feel lightheaded after taking it at night. If donepezil causes dreams or disrupts your sleep pattern, you could consider taking it in the morning instead.
Stepwise Dosage Escalation to Minimize Side Effects
Gradual titration reduces the risk of nausea, diarrhea, and other adverse effects, improving patient comfort.
Guidelines for Taking Orally Disintegrating Tablets
Place the tablet on the tongue; it dissolves quickly without water. Ideal for patients with swallowing difficulties.
Administration Timing and Adherence Tips
Take once daily, preferably in the evening. Setting reminders aids in adherence to the regimen.
Adjustments for Special Populations
Dosage adjustments may be required for patients with hepatic or renal impairment. Close monitoring is essential.
Storage Guidelines for Aricept Evess
Optimal Temperature and Humidity Conditions
Store at room temperature (15-30°C) in a dry, cool environment. Avoid exposure to excessive moisture.
Safe Handling and Disposal
Keep out of reach of children. Dispose of expired tablets responsibly to prevent misuse.
Shelf-Life and Stability Considerations
Observe the expiration date for optimal efficacy. Improper storage can degrade the medication's quality.
Donepezil hydrochloride side effects
Common Side Effects
Less Common Side Effects
- Bradycardia
- Gastrointestinal bleeding
Rare but Serious Adverse Reactions
Includes severe allergic reactions and heart block. Immediate medical attention is crucial.
Drug Interactions
Interaction with Other Cholinesterase Inhibitors
Combining with similar drugs can amplify cholinergic effects, risking toxicity.
Impact of Anticholinergic Medications
The effectiveness of Aricept Evess therapeutic benefits is diminished when counteracted.
Cautions with NSAIDs and Cardiovascular Drugs
Increases the risk of gastrointestinal bleeding when used with NSAIDs. Monitor interactions with beta-blockers.
Alcohol and Dietary Considerations
Alcohol can exacerbate side effects like drowsiness. A balanced diet supports overall health and treatment effectiveness.
Warnings and Contraindications
Contraindications for Hypersensitivity to Donepezil
Aricept Evess is contraindicated in individuals with a known hypersensitivity to donepezil hydrochloride or any of its excipients. Hypersensitivity reactions may manifest as severe dermatological conditions, respiratory distress, or anaphylaxis, necessitating immediate discontinuation and intervention.

Risks in Patients with Peptic Ulcer Disease or Asthma
Patients with a history of peptic ulcer disease are at heightened risk of gastrointestinal complications. Similarly, individuals with asthma or chronic obstructive pulmonary disease should exercise caution, as cholinesterase inhibitors can exacerbate respiratory conditions.
QT Prolongation and Cardiac Risks
Aricept Evess may contribute to QT prolongation, a cardiac arrhythmia that can precipitate life-threatening events such as Torsades de Pointes. Regular electrocardiographic monitoring is advisable, especially in patients with predisposing factors.
Situations Warranting Discontinuation
- Severe gastrointestinal bleeding.
- Uncontrolled bradycardia.
- Intolerable side effects or allergic reactions.
Precautions for Administration
Importance of Medical History Evaluation
A thorough medical history is imperative before initiating treatment with Aricept Evess. Conditions such as epilepsy, hepatic impairment, or renal dysfunction may necessitate tailored dosing or enhanced monitoring.
Monitoring During Treatment
Continuous monitoring ensures the early detection of adverse effects or efficacy loss. Regular follow-ups with cognitive assessments and laboratory evaluations can guide therapeutic adjustments.
Risks of Abrupt Discontinuation
Stopping Aricept Evess suddenly may result in a rapid decline in cognitive function. Gradual tapering under medical supervision is recommended to mitigate withdrawal effects.
Recognizing and Addressing Potential Allergic Reactions
Early signs of allergic reactions, such as rash or facial swelling, should prompt immediate medical consultation. Delayed treatment can lead to severe complications.
Donepezil hydrochloride pregnancy
There is no data concerning the impact of donepezil, on infants; therefore it is advised to avoid its use during pregnancy.
Administration to Elderly Patients
Efficacy and Safety Considerations in Geriatric Populations
Elderly individuals frequently experience advantages from using Aricept Evess medication. May also be more prone, to side effects because of physiological changes related to aging.
Monitoring for Polypharmacy Interactions
Elderly individuals frequently use multiple medications, increasing the risk of drug interactions. Careful evaluation of concomitant therapies is essential to avoid adverse outcomes.
Managing Comorbid Conditions
Conditions like diabetes or hypertension must be managed concurrently. The synergistic impact of these conditions and cognitive decline necessitates a holistic therapeutic approach.
Administration to Pregnant Women and Nursing Mothers
Safety Profile in Pregnancy (Category C)
Animal studies suggest potential risks to the fetus, categorizing Aricept Evess as a Category C drug. Its use during pregnancy should be limited to cases where the potential benefits outweigh the risks.
Risk vs. Benefit Analysis
Clinicians must weigh the need for cognitive symptom management against the possible teratogenic effects. A shared decision-making approach is crucial.
Potential Impact on Lactation and Nursing Infants
It is unclear if donepezil passes into breast milk. Nursing mothers should discuss the potential risks with their healthcare providers.

Administration to Children
Limited Data on Pediatric Use
Currently, there is minimal clinical evidence supporting the use of Aricept Evess in pediatric populations. The safety and efficacy remain largely uncharted.
Investigational Use for Developmental and Cognitive Disorders
Emerging studies are exploring its role in managing developmental disorders, such as attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder. These applications remain experimental.
Ethical Considerations in Pediatric Trials
Conducting trials in children requires stringent ethical oversight to ensure the safety and well-being of participants.
Overdosage and Management
Symptoms of Donepezil Overdose
Overdose symptoms include severe nausea, excessive salivation, bradycardia, hypotension, and seizures. These symptoms demand immediate medical intervention.
Severe Nausea, Bradycardia, Seizures
Bradycardia and seizures are particularly concerning as they can lead to life-threatening complications. Prompt identification and treatment are paramount.
Emergency Protocols for Overdose Treatment
- Administration of activated charcoal to limit absorption.
- Intravenous fluids to stabilize blood pressure.
- Symptomatic treatment for seizures and cardiac irregularities.
Role of Atropine as an Antidote
Atropine, a cholinergic antagonist, is the antidote of choice. It reverses the cholinergic effects of donepezil overdose effectively.
Handling Precautions for Aricept Evess
Safe Handling to Avoid Contamination
Aricept Evess should be stored in its original packaging until use to prevent contamination. Always handle with clean, dry hands.
Guidelines for Caregivers Administering the Medication
Caregivers should ensure the patient consumes the tablet entirely. For orally disintegrating tablets, confirm dissolution before administering additional liquids or food.
Proper Disposal of Unused or Expired Medication
Unused or expired medication should be disposed of according to local pharmaceutical waste guidelines. Avoid flushing medications unless specified.
Aricept Evess FAQ
- Donepezil when to use?
- Donepezil when to stop?
- Donepezil when to take?
- Donepezil what does it do?
- Donepezil what to monitor?
- What donepezil used for?
- What donepezil treat?
- Donepezil how to take?
- Donepezil how long to take effect?
- How donepezil hydrochloride works?
- How donepezil works?
- Can donepezil cause diarrhea?
- Can donepezil cause anger?
- Can donepezil and memantine be taken together?
- Can donepezil be taken in the morning?
- What are donepezil hydrochloride tablets for?
- Are donepezil and memantine the same?
- How long will donepezil be effective?
- How long will donepezil work?
- Will donepezil help with memory loss?
- Why donepezil at night?
- Why is donepezil taken in morning?
- Why is donepezil prescribed?
- Which is better donepezil vs rivastigmine?
- Which is better donepezil or memantine?
- Donepezil which group?
- Where does donepezil work?
- Donepezil when to use?
- Donepezil when to stop?
- Donepezil when to take?
- Donepezil what does it do?
- Donepezil what to monitor?
- What donepezil used for?
- What donepezil treat?
- Donepezil how to take?
- Donepezil how long to take effect?
- How donepezil hydrochloride works?
- How donepezil works?
- Can donepezil cause diarrhea?
- Can donepezil cause anger?
- Can donepezil and memantine be taken together?
- Can donepezil be taken in the morning?
- What are donepezil hydrochloride tablets for?
- Are donepezil and memantine the same?
- How long will donepezil be effective?
- How long will donepezil work?
- Will donepezil help with memory loss?
- Why is donepezil taken in morning?
- Why is donepezil prescribed?
- Which is better donepezil vs rivastigmine?
- Which is better donepezil or memantine?
- Where does donepezil work?
Donepezil when to use?
Donepezil is commonly given to people diagnosed with Alzheimer's disease to help them cope with the effects of memory loss and declining cognitive abilities associated with the condition.
Donepezil when to stop?
Continue taking donepezil even if you feel well, and always seek advice from your doctor before stopping its use. Your doctor could start you off with a dosage of donepezil and then adjust it after 4 to 6 weeks; further changes may be made after 3 months or longer.
Donepezil when to take?
It's generally suggested that you take your donepezil before going to bed because it may occasionally result in dizziness after you've taken the medication. If you experience dreams or have trouble falling asleep after taking donepezil at night, it could be worth thinking about switching to mornings.
Donepezil what does it do?
Donepezil is a medication prescribed to relieve symptoms linked to Alzheimer's disease, like memory loss and disorientation.
Donepezil what to monitor?
Remember to check your heart rate before you start anything. If it's over 60 beats per minute, an ECG might be wise before beginning any treatment.
What donepezil used for?
Donepezil is commonly recommended to help with memory loss and cognitive changes associated with stages of Alzheimer's disease.
What donepezil treat?
Donepezil is commonly prescribed to treat dementia associated with stages of Alzheimer's disease, whether it is mild or severe, which is marked by memory loss and changes in function.
Donepezil how to take?
Donepezil is offered in tablet form. It is a dissolving, disintegrating tablet that quickly melts in the mouth. It is usually taken once a day, at night before bedtime, either with or without food.
Donepezil how long to take effect?
It usually takes a month for donepezil to start exhibiting its effects.
How donepezil hydrochloride works?
Donepezil works by slowing down the breakdown of a chemical called acetylcholine in the brain.
How donepezil works?
Donepezil works by preventing the breakdown of a brain neurotransmitter called acetylcholine.
Can donepezil cause diarrhea?
Some individuals might experience nausea and upset stomach as episodes of diarrhea.
Can donepezil cause anger?
The negative impacts noticed included decreased appetite (anorexia), sickness (nausea), upset stomach (diarrhea), restlessness and irritability (agitation), frustration and hostility (anger), dizziness (lightheadedness), false beliefs or perceptions (delusions), trouble sleeping (insomnia) and inability to unwind.
Can donepezil and memantine be taken together?
Doctors often recommend the use of memantine and donepezil together to help with memory loss and cognitive decline in individuals suffering from moderate to severe Alzheimer's disease.
Can donepezil be taken in the morning?
If you find that taking donepezil leads to experiencing dreams or trouble sleeping at night and want to make a change in your routine for sleep quality and dream recall patterns, consider shifting your dosage to the morning rather than at bedtime.
What are donepezil hydrochloride tablets for?
Donepezil is a drug that helps in treating types of dementia but does not offer a solution for the illness itself. It works well in alleviating symptoms linked to Alzheimers disease, Parkinsons disease, and Lewy body dementia. It could also be helpful for people dealing with dementia.
Are donepezil and memantine the same?
Donepezil increases acetylcholine levels in synapses to enhance transmission efficiency, and memantine interferes with the flow in NMDA receptor channels, which is important for brain function.
How long will donepezil be effective?
Over the course of years, Donepezil—commonly referred to as Aricept—has demonstrated efficacy in treating mild to moderate Alzheimer's disease and Lewy body dementia.
How long will donepezil work?
Donepezil, also known as Aricept or Adlyarity, is a medication often prescribed for managing dementia associated with Alzheimer's disease. The effects of this medication may not be immediately apparent and could take weeks to months to exhibit benefits; however, it has the potential to remain effective for years.
Will donepezil help with memory loss?
Donepezil is part of a category of medications called cholinesterase inhibitors. These medications help improve functions such as memory and focus in people by increasing the levels of a natural substance in the brain that supports clear thinking and better communication skills.
Why donepezil at night?
A side effect is drowsiness, which is good at night.
Why is donepezil taken in morning?
If using donepezil results in experiencing dreams or having trouble sleeping.
Why is donepezil prescribed?
Assist in handling memory difficulties and confusion (referred to as dementia) for those with Alzheimer's disease.
Which is better donepezil vs rivastigmine?
Rivastigmine is more effective
Which is better donepezil or memantine?
Individuals with Alzheimer's disease are often prescribed Donepezil for treatment purposes; on the other hand, memantine is usually suggested for those experiencing more severe forms of the condition.
Donepezil which group?
Cholinesterase inhibitor
Where does donepezil work?
Donepezil works by slowing down the breakdown of a substance called acetylcholine in the brain, which helps with nerve cell communication, in the brain.
Donepezil when to use?
Individuals with Alzheimer's disease are often given Donepezil to help them cope with the symptoms of dementia, which is a condition affecting memory and cognitive abilities.
Donepezil when to stop?
Continue taking donepezil even if you feel well, and always consult your doctor before stopping it. Your doctor may start you off with a dose of donepezil and then adjust it after 4 to 6 weeks; further changes can be made after 3 months or longer.
Donepezil when to take?
It's generally advised to take your dose of donepezil before going to bed, as it may occasionally result in dizziness following the intake of the medication. If you experience dreams or trouble falling asleep after taking donepezil at night, you might want to think about switching to mornings for administration.
Donepezil what does it do?
Donepezil is a medication prescribed to assist in reducing the symptoms linked to Alzheimer's disease, like memory decline and disorientation.
Donepezil what to monitor?
Remember to check your heart rate before you start anything! If it's above 60 beats per minute, it's wise to have an ECG done before beginning any treatment.
What donepezil used for?
Donepezil is commonly prescribed to help with memory loss and cognitive changes associated with stages of Alzheimer's disease.
What donepezil treat?
Donepezil is commonly used to treat dementia associated with stages of Alzheimer's disease, whether it's mild or severe, which is marked by memory loss and changes in function.
Donepezil how to take?
Donepezil comes in tablet form and is a dissolving tablet that dissolves swiftly in the mouth. It is taken daily with or without food and usually at night before bedtime.
Donepezil how long to take effect?
It usually takes a month for the effects of donepezil to become noticeable.
How donepezil hydrochloride works?
Donepezil works by slowing down the breakdown of a chemical called acetylcholine in the brain.
How donepezil works?
Donepezil works by preventing the breakdown of a neurotransmitter called acetylcholine in the brain.
Can donepezil cause diarrhea?
Some people might experience nausea and an upset stomach, with bouts of diarrhea.
Can donepezil cause anger?
The negative outcomes noted included decreased appetite, feelings of queasiness, stomach discomfort, restlessness and annoyance, frustration and hostility, dizziness, false beliefs or perceptions, trouble sleeping, and difficulty unwinding.
Can donepezil and memantine be taken together?
Patients suffering from moderate to severe Alzheimer's disease have often been prescribed a combination of memantine. Donepezil to help manage memory loss and cognitive changes associated with the condition.
Can donepezil be taken in the morning?
If you find that taking donepezil leads to experiencing dreams or trouble sleeping during the night, you might consider taking it in the morning before bedtime.
What are donepezil hydrochloride tablets for?
Donepezil is a drug that helps in treating types of dementia but does not offer a solution to the condition itself. It is helpful in dealing with the symptoms linked to Alzheimers disease, Parkinsons disease, and Lewy body dementia. It might also be advantageous for people suffering from dementia.
Are donepezil and memantine the same?
Donepezil functions by increasing the amount of acetylcholine in the synapses to enhance communication, while memantine interferes with the passage of current through NMDA receptor channels that are involved in brain function.
How long will donepezil be effective?
Donepezil, commonly referred to as Aricept, has demonstrated its efficacy in treating mild to moderate cases of Alzheimer's disease and dementia with Lewy bodies over an extended period of time.
How long will donepezil work?
Donepezil is a medication prescribed under the names Aricept or Adlyarity to treat dementia associated with Alzheimer's disease; however, its effects may not be immediately evident. It could gradually improve over a span of weeks to months and potentially maintain effectiveness for years.
Will donepezil help with memory loss?
Donepezil is part of a class of medications called cholinesterase inhibitors. These medications help improve functions such as memory and focus by increasing the levels of a substance in the brain that supports clear thinking and communication effectiveness.
Why is donepezil taken in morning?
If you are using donepezil and experiencing dreams or trouble sleeping, please consult with your healthcare provider for guidance.
Why is donepezil prescribed?
Assist in handling memory issues and confusion (referred to as dementia) in people with Alzheimer's disease.
Which is better donepezil vs rivastigmine?
Rivastigmine is more effective
Which is better donepezil or memantine?
Donepezil is often used to treat people with Alzheimer's disease, and memantine is usually suggested for those with moderate cases of the condition.
Where does donepezil work?
Donepezil works by slowing down the breakdown of a substance called acetylcholine in the brain which helps nerve cells communicate with each other effectively.