Decapeptyl Depot
- I. Introduction to Decapeptyl Depot
- II. Approved Uses of Decapeptyl Depot
- III. Off-Label Uses of Decapeptyl Depot
- IV. Dosage and Administration of Decapeptyl Depot
- V. Composition and Formulation of Decapeptyl Depot
- VI. How Decapeptyl Depot Works
- VII. Side Effects of Decapeptyl Depot
- VIII. Common Side Effects of Decapeptyl Depot
- IX. Serious Side Effects and Complications
- X. Drug Interactions with Decapeptyl Depot
- XI. Warnings and Precautions for Decapeptyl Depot
- XII. Contraindications for Decapeptyl Depot
- XIII. Careful Administration of Decapeptyl Depot
- XIV. Important Precautions to Consider
- XV. Administration to Elderly Patients
- XVI. Administration to Pregnant Women and Nursing Mothers
- XVII. Administration to Children
- XVIII. Overdose and What to Do
- XIX. Handling and Storage of Decapeptyl Depot
I. Introduction to Decapeptyl Depot
What is Decapeptyl Depot?
Decapeptyl Depot is a type of medicine mainly employed for treating hormone-related issues by adjusting hormone levels in the body using a component called triptorelin from the GnRH (gonadotropin-releasing hormone) agonists drug group in medical situations to ensure effective treatment outcomes where hormone regulation is key.
Overview of the Active Ingredient: Triptorelin
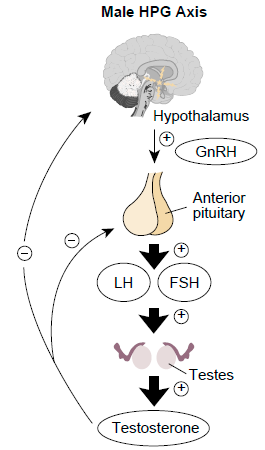
Triptorelin is the component found in Decapeptyl Depot. It is designed to effectively mimic GnRH in a synthetic form. It plays a role as a GnRH agonist by impacting the release of various hormones, like luteinizing hormone (LH) and follicle-stimulating hormone (FSH), which play crucial roles in maintaining the reproductive system's balance. Through its influence on the hypothalamic-pituitary-gonadal axis, triptorelin helps regulate the production of sex hormones such as estrogen and testosterone.
Therapeutic Class and Drug Profile
Decapeptyl Depot is an endocrine drug classified as a GnRH agonist. It is commonly used to treat hormone-related conditions such as prostate cancer and endometriosis. It works by triggering and later suppressing the production of reproductive hormones through controlled release in the body.
Mechanism of Action: How Decapeptyl Depot Works
Triptorelin found in Decapeptyl Depot functions by acting like GnRH and attaching to GnRH receptors in the gland to stimulate a surge in sex hormone production (testosterone in males and estrogen in females). Over time of use though it leads to a feedback loop that ultimately inhibits the production of these hormones—a process beneficial for conditions fueled by an overabundance of hormones, like prostate cancer and endometriosis.
II. Approved Uses of Decapeptyl Depot
Treatment of Prostate Cancer
Decapeptyl Depot is approved for use in the management of prostate cancer, particularly in patients with advanced or metastatic forms of the disease. By lowering testosterone levels, it helps reduce tumor growth and alleviate symptoms associated with prostate cancer. The suppression of testosterone can significantly improve quality of life and may slow disease progression.
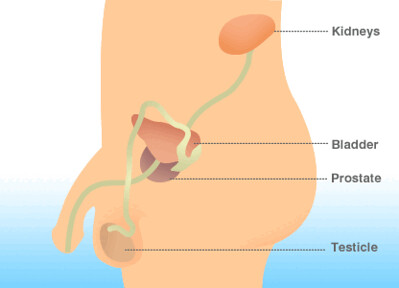
Management of Endometriosis
Endometriosis is a health issue in which tissue resembling the lining of the uterus develops outside the uterus itself. It is commonly managed using Decapeptyl Depot medication to lower estrogen production, which triggers the growth of this tissue outside the uterus walls. Reducing estrogen levels with this drug treatment aids in easing pain and reducing the tissue size, leading to relief from symptoms.
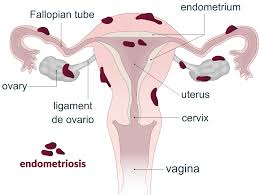
Use in Uterine Fibroids
Decapeptyl Depot is also prescribed to manage fibroids. Growths in the uterus lead to heavy bleeding and discomfort by inhibiting estrogen production to shrink the fibroids and alleviate symptoms without necessarily requiring surgery.
Role in Precocious Puberty (Children)
Use in Assisted Reproductive Technology (ART)
Decapeptyl Depot is also utilized in reproductive assisted technology (ART), such as in vitro fertilization (IVF). It assists in regulating ovulation timing by inhibiting the body's natural hormone cycle and improving the management of the stimulation process in fertility treatments.
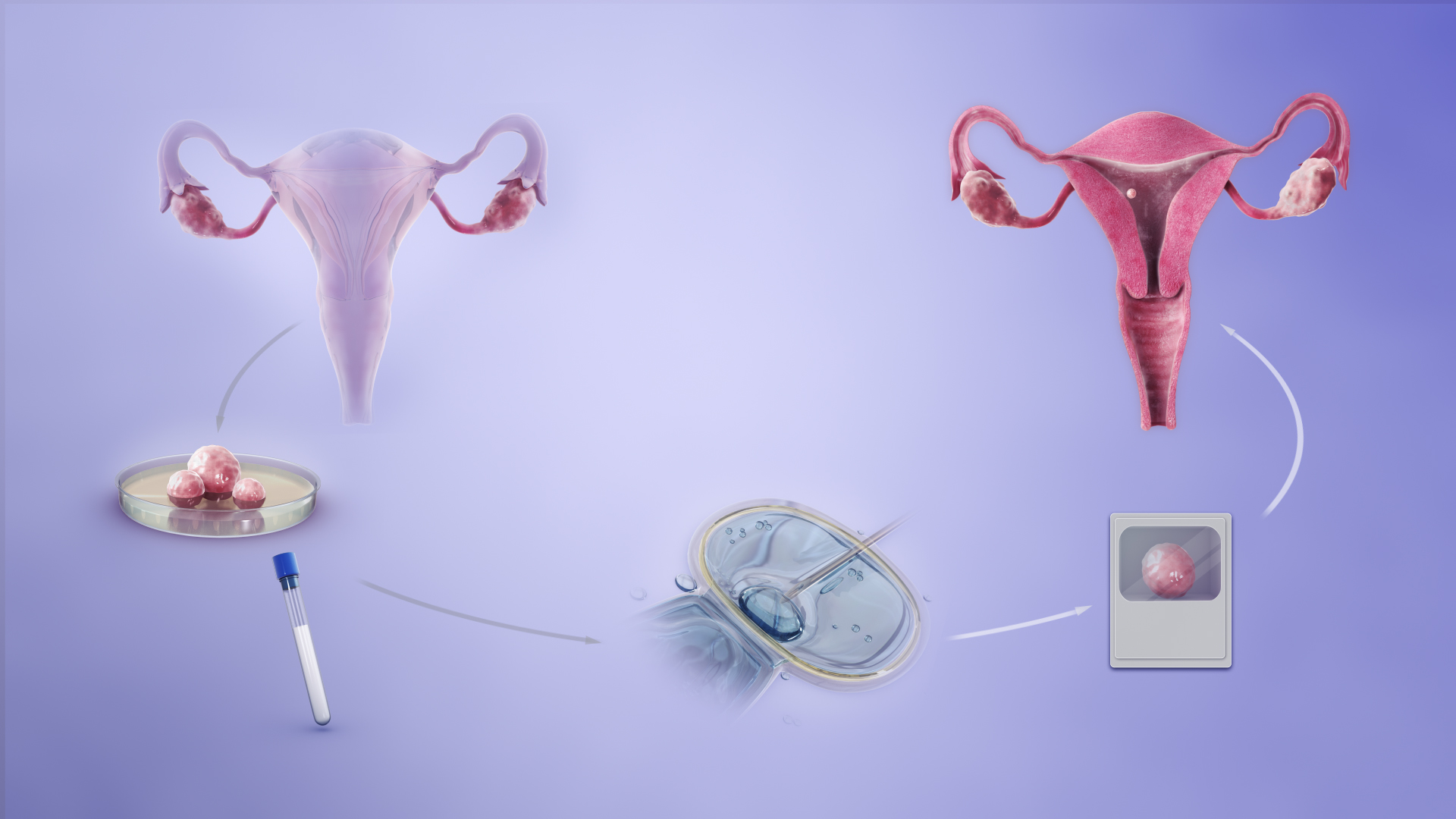
III. Off-Label Uses of Decapeptyl Depot
Treatment of Breast Cancer in Women
Although it's not formally endorsed for this purpose by authorities, Decapeptyl Depot has been utilized off-label to address hormone breast cancer in women before the menopause stage. The medication works by lowering estrogen levels which can slow down the growth of tumors that rely on estrogen for development
Use in Managing Hormone-Sensitive Conditions
Decapeptyl Depot is occasionally prescribed for purposes not officially approved to treat hormone-related issues, like gynecologic cancers and endometrial hyperplasia, by regulating hormone levels to slow down disease advancement.
Role in Transgender Hormone Therapy
Decapeptyl Depot has been used in transgender hormone therapy to suppress endogenous puberty in transgender adolescents and facilitate the desired feminization or masculinization process in adults. It plays a critical role in creating a more supportive environment for gender-affirming hormone treatments.
Off-Label Use in Polycystic Ovary Syndrome (PCOS)
Decapeptyl Depot has shown potential in the off-label treatment of polycystic ovary syndrome (PCOS), a condition often associated with hormonal imbalances. By reducing elevated androgen levels, it can help manage symptoms such as hirsutism and acne.
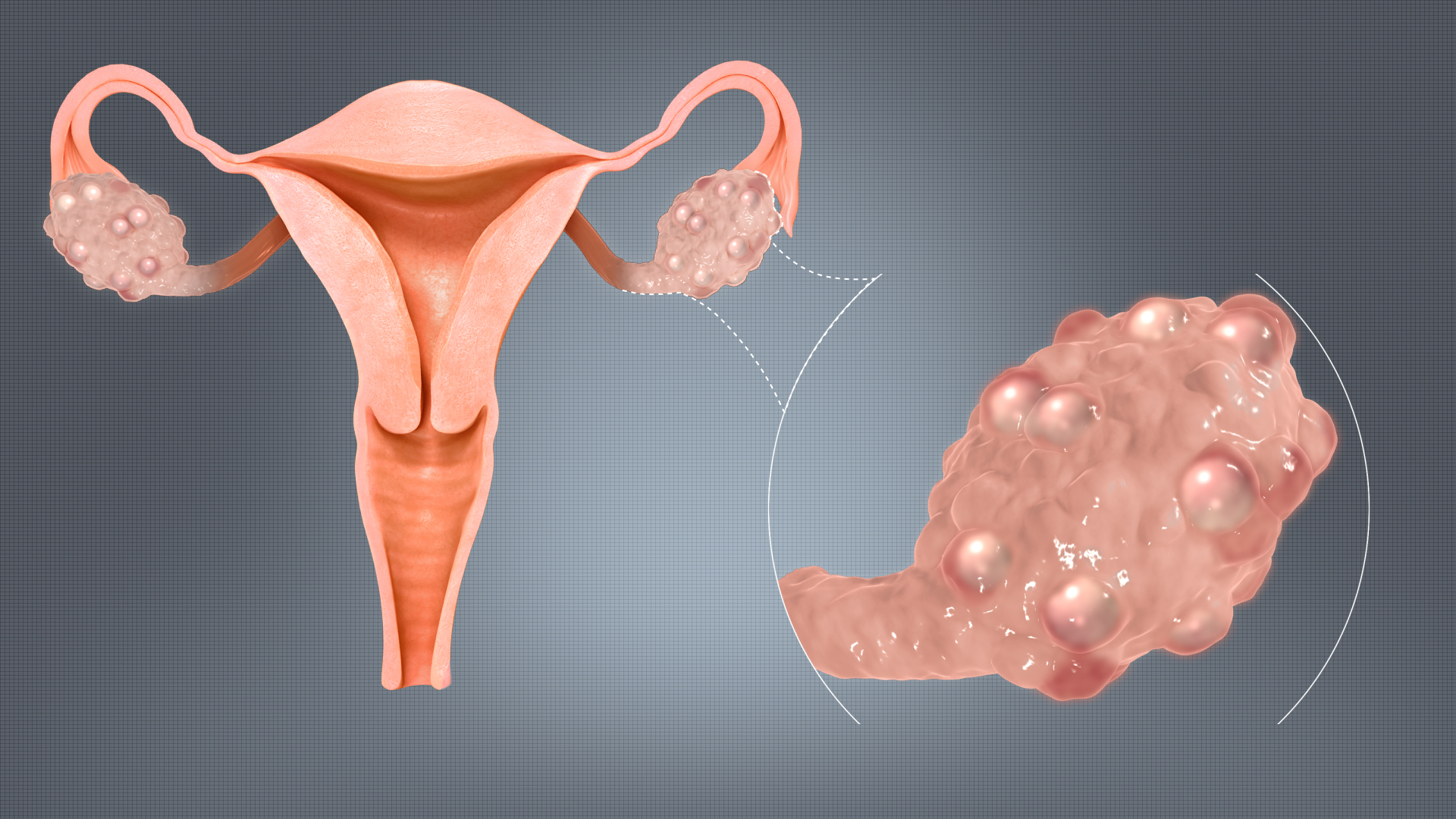
Impact on Chronic Pelvic Pain Syndrome
Decapeptyl Depot has been employed in instances to relieve pelvic discomfort associated with conditions like endometriosis or other gynecological issues by decreasing estrogen levels to alleviate pain caused by endometrial-like tissue stimulation.
IV. Dosage and Administration of Decapeptyl Depot
Standard Dosage Recommendations for Different Conditions
The dosage of Decapeptyl Depot varies depending on the condition being treated. For prostate cancer, the typical dosage is 11.25 mg every 3 months. For endometriosis and uterine fibroids, the dosage is typically 3.75 mg monthly or 11.25 mg every 3 months. Precocious puberty is often treated with 3.75 mg every 4 weeks.
How Decapeptyl Depot is Administered (Injectable Depot Form)
Decapeptyl Depot is usually given through a shot into the muscles of the buttock or thigh area for release of medication to maintain therapeutic levels without the need for frequent injections over time.
Frequency of Administration for Various Indications
The dosing schedule varies based on the condition being addressed; for instance, in the case of prostate cancer, treatment every 3 months may be necessary, whereas for conditions like endometriosis and uterine fibroids, monthly or quarterly injections might be needed.
Preparation and Reconstitution of the Injection
To use Decapeptyl Depot, it needs to be mixed with a solvent, as per the directions included in the package insert. Once mixed, the solution should be promptly injected.
Special Instructions for Injection Site and Technique
The healthcare provider should give the shot using equipment, change the injection spot to avoid irritating the tissue, and administer the injection slowly for better absorption.
V. Composition and Formulation of Decapeptyl Depot
Active Ingredients: Triptorelin Acetate
The main component in Decapeptyl Depot is triptorelin acetate, a GnRH analog that affects the pituitary gland and helps control the release of gonadal hormones.
Inactive Ingredients and Excipients
Decapeptyl Depot includes active components, like mannitol and sodium chloride, along with water, for injection to stabilize the formula and aid in the gradual release of the main ingredient.
Formulation of Decapeptyl Depot (Injectable Suspension)
Decapeptyl Depot comes in a suspension that helps release triptorelin gradually over time, lessening the necessity for frequent injections.
Available Strengths and Concentrations
There are accessible strengths of Decapeptyl Depot, such as 3.75 mg, 11.25 mg, and 22.50 mg, depending on the specific condition being addressed and how often it needs to be administered.
VI. How Decapeptyl Depot Works
Mechanism of Action as a GnRH Agonist
Decapeptyl Depot works as a GnRH agonist by triggering the gland to release high levels of gonadotropins like LH and FSH; yet, with prolonged use, it eventually lowers the activity of GnRH receptors, leading to a significant decrease in the production of sex hormones by the gonads.
Role in Reducing Gonadal Hormone Production
Decapeptyl Depot is successful in treating hormone illnesses by inhibiting the secretion of sex hormones like testosterone and estrogen, which are particularly advantageous in managing conditions such as prostate cancer and endometriosis.
Impact on the Hypothalamic-Pituitary-Gonadal Axis
Decapeptyl Depot exerts its effects by influencing the hypothalamic-pituitary-gonadal axis. This regulatory pathway controls the release of hormones that govern reproductive processes. By blocking this pathway, Decapeptyl Depot helps manage conditions associated with excessive hormone production.
Effect on Testosterone and Estrogen Levels in the Body
Lowered testosterone levels in men and reduced estrogen levels in women can occur when gonadal hormone production is suppressed. In men, this might cause prostate tumor size reduction, while in women, it could help alleviate symptoms associated with conditions such as endometriosis.
Differences Between Decapeptyl Depot and Other GnRH Analogs
Among the GnRH analogs available in the market, Decapeptyl Depot stands out due to its formulation and extended duration of action. It effectively maintains hormone levels over a prolonged period with administration requirements making it a convenient choice, for individuals undergoing long term treatment.
VII. Side Effects of Decapeptyl Depot
Overview of Potential Side Effects
Like all medications, Decapeptyl Depot can cause side effects. While most are mild and temporary, some may require medical attention. These side effects can range from common reactions like injection site pain to more serious issues like bone density loss.
Short-Term vs. Long-Term Side Effects
Common immediate reactions may consist of experiencing waves of heat and tiredness, as well as sensitivity at the injection site area. Over time, potential consequences could entail a decrease in bone strength that necessitates observation. The intensity of these effects can differ based on how long the treatment lasts.
VIII. Common Side Effects of Decapeptyl Depot
Hot Flashes and Night Sweats
Hot flashes and night sweats are often experienced as a side effect of Decapeptyl Depot treatment in women dealing with endometriosis or uterine fibroids due to the decrease in estrogen levels.
Decreased Libido and Erectile Dysfunction
For male patients, Decapeptyl Depot can lead to a significant decrease in libido and erectile dysfunction due to the suppression of testosterone levels. These symptoms may improve once treatment is completed.
Injection Site Reactions (Pain, Swelling, Irritation)
People often have reactions at the spot where Decapeptyl Depot is injected, like pain or swelling. They may also feel irritated there, but these usually aren't too bad and go away by themselves.
Fatigue and Mood Changes
Patients may also experience fatigue and mood changes during treatment with Decapeptyl Depot. These effects are often temporary and improve as the body adjusts to the hormonal changes induced by the drug.
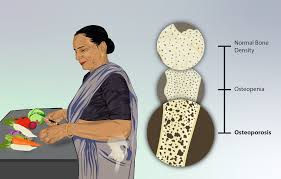
Bone Density Loss and Osteoporosis Risk
Prolonged use of Decapeptyl Depot may decrease bone density and raise the likelihood of developing osteoporosis; thus, it is advisable to monitor bone health throughout extended treatment periods.
IX. Serious Side Effects and Complications
Risk of Cardiovascular Events (e.g., Heart Attack, Stroke)
Although rare, Decapeptyl Depot may increase the risk of cardiovascular events, particularly in individuals with pre-existing heart conditions. Regular monitoring and risk assessment are advised.
Allergic Reactions and Anaphylaxis
Severe allergic reactions, including anaphylaxis, can occur, though these are uncommon. Symptoms of an allergic reaction include swelling, difficulty breathing, and rash. Immediate medical attention is required if these occur.
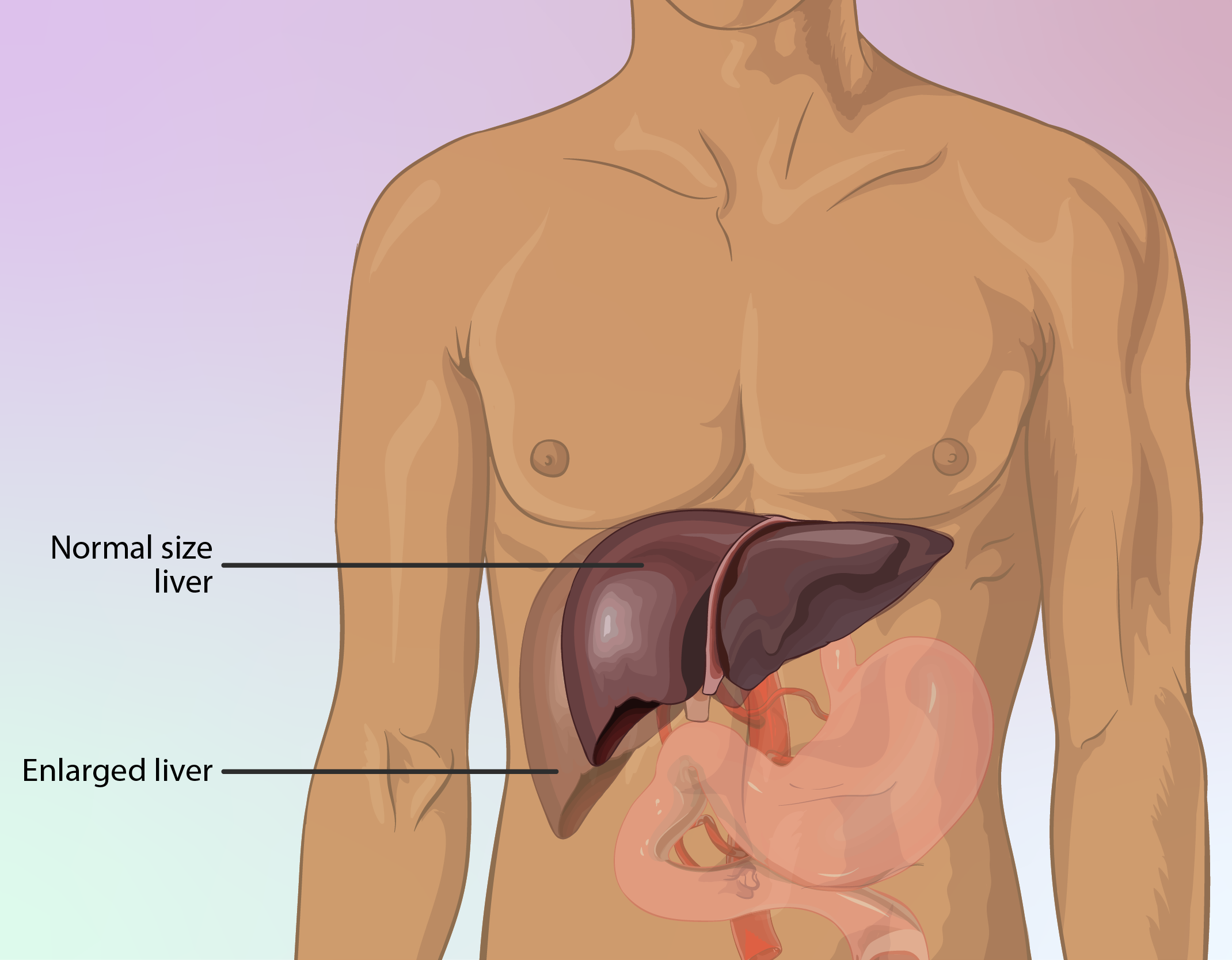
Impact on Liver and Kidney Function
In some cases, Decapeptyl Depot may affect liver and kidney function. For patients with underlying hepatic or renal conditions, monitoring liver enzymes and kidney function tests during treatment is recommended.
Long-Term Effects on Fertility and Reproductive Health
Long-term use of Decapeptyl Depot can impact fertility, particularly in both men and women. While the effects may be reversible after discontinuation, fertility should be discussed with a healthcare provider before starting therapy.
X. Drug Interactions with Decapeptyl Depot
Potential Drug Interactions with Other Hormone Therapies
Decapeptyl Depot, a GnRH agonist, may interact with other hormone therapies. It is essential to evaluate the concomitant use of medications such as oral contraceptives, estrogen, and testosterone replacement therapy. These drugs can alter the effectiveness of Decapeptyl Depot, as they may counteract its suppression of gonadal hormones. In particular, estrogen and testosterone therapies could potentially interfere with Decapeptyl Depot's action, diminishing its therapeutic benefits.
Interaction with Anticoagulants and Blood Thinners
Patients receiving Decapeptyl Depot and anticoagulants, such as warfarin or direct oral anticoagulants, should be monitored closely. Hormonal therapies can influence the coagulation system, possibly enhancing the effects of blood thinners, which could lead to an increased risk of bleeding. Adjustments to anticoagulant dosing may be necessary to avoid complications.
Effects on Other Medications for Prostate Cancer
For individuals receiving Decapeptyl Depot in conjunction with other prostate cancer treatments, such as androgen deprivation therapy (ADT) or chemotherapy, there may be a cumulative effect in lowering testosterone levels. This could increase the risk of side effects like fatigue, muscle weakness, and sexual dysfunction. Close monitoring by the healthcare provider is recommended to manage these combined therapies effectively.
Interaction with Immunosuppressants
Immunosuppressive drugs, including corticosteroids and medications used in organ transplantation, may also interact with Decapeptyl Depot. The hormone suppression caused by Decapeptyl Depot can alter the immune response, potentially diminishing the effects of immunosuppressants. It is important to assess any potential need for dose adjustments or closer monitoring of immune function during concurrent use.
Impact on Medications Affecting Bone Density
Long-term use of Decapeptyl Depot can have a detrimental impact on bone mineral density, increasing the risk of osteoporosis. When used with other medications that affect bone health, such as corticosteroids or other agents that may reduce bone density, there is a compounded risk of fractures and bone weakening. Regular monitoring of bone health and appropriate interventions are necessary when these drugs are used together.
XI. Warnings and Precautions for Decapeptyl Depot
General Warnings for Use in Specific Populations
Decapeptyl Depot should be used with caution in specific populations, including those with a history of cardiovascular disease, liver or kidney disorders, and psychiatric conditions. These individuals may be more susceptible to side effects or adverse reactions, necessitating careful assessment before beginning treatment. Regular monitoring is advised to ensure the safety of the patient during therapy.
Potential for Pituitary Apoplexy with GnRH Agonists
The application of GnRH agonists such as Decapeptyl Depot has been linked to a severe condition called apoplexy—a sudden hemorrhage or infarction of the pituitary gland that can lead to symptoms like intense headaches and visual disturbances along with nausea and changes in consciousness levels, requiring prompt medical attention.
Risk of Hypersensitivity Reactions
While it's not common, Decapeptyl Depot may lead to hypersensitivity responses, like anaphylaxis, skin irritations, and swelling. If there are indications of a reaction, it's crucial to stop using the medication right away and seek urgent medical help. Healthcare professionals need to be ready for reactions. Carefully watch for any signs of hypersensitivity, especially following the initial dose.
Effects on Mood and Psychological Health
Decapeptyl Depot may lead to impacts on individuals undergoing prolonged treatment with the medication. Affected patients might encounter shifts in mood or feelings of depression and anxiety to changes resulting from the inhibition of sex hormones. It is advised that counseling and emotional assistance be provided as needed and that patients be observed for any indications of health decline throughout the course of treatment.
XII. Contraindications for Decapeptyl Depot
Contraindicated in Pregnancy and Lactation
Decapeptyl Depot should not be taken while pregnant because it could interfere with natural hormone processes and potentially harm the developing fetus. During breastfeeding it's best to avoid using this medication as it can pass into breast milk and impact the hormonal balance of the baby. If you are pregnant or nursing it is advisable to explore treatment options.
Conditions Where Decapeptyl Depot Should Not Be Used (e.g., Certain Types of Cancers)
Decapeptyl Depot should not be administered to individuals with cancer types that do not respond to hormones or have a source of origin; it should be avoided in situations where reducing gonadal hormones might worsen the condition, like in cases of pituitary tumors or untreated adrenal insufficiency. It is essential to conduct a comprehensive clinical assessment prior to starting the treatment regimen.
Contraindicated in Individuals with Hypersensitivity to Triptorelin or Other Components
Individuals who have demonstrated hypersensitivity to triptorelin or any excipients in the formulation of Decapeptyl Depot should avoid its use. Hypersensitivity reactions can range from mild skin rashes to severe anaphylactic reactions. Such patients should be prescribed an alternative treatment that does not contain triptorelin.
XIII. Careful Administration of Decapeptyl Depot
Monitoring Requirements During Therapy
Patients undergoing treatment with Decapeptyl Depot should be closely monitored to assess the effectiveness and detect any adverse effects. This includes regular hormone level assessments to confirm the suppression of testosterone or estrogen and periodic imaging studies to evaluate the condition being treated.
Importance of Periodic Blood Tests (e.g., Hormone Levels)
Regular blood tests are necessary to monitor the levels of gonadal hormones, like testosterone and estrogen, to ensure that Decapeptyl Depot is working as intended and that hormone suppression is effective enough. They also help spot any irregularities that might suggest a reaction to the treatment.
Special Considerations for Patients with Liver or Kidney Disease
Patients who have liver or kidney issues need to be careful when taking Decapeptyl Depot. These organs play a role in how the drug is processed and removed from the body; if their function is not optimal, it could affect the drug's levels in the system. Patients with liver or kidney problems may require changes in their dosage and should be closely watched during their treatment journey.
Administration Precautions in Elderly Patients
Elderly patients may be more susceptible to the adverse effects of Decapeptyl Depot, particularly regarding bone health and cardiovascular risk. Close monitoring and potential dose adjustments should be considered to minimize the risks of fractures, osteoporosis, and cardiovascular events.
XIV. Important Precautions to Consider
Risk of Tumor Flare and Its Management
In some patients, particularly those with prostate cancer, Decapeptyl Depot may initially cause a "tumor flare" before the suppression of testosterone takes effect. This can temporarily worsen symptoms such as bone pain or urinary obstruction. Supportive measures, including the use of anti-androgens, may be necessary to manage these effects during the first weeks of treatment.
Caution in Patients with Cardiovascular Disease
Decapeptyl Depot should be used with caution in patients with cardiovascular disease. The drug can potentially increase the risk of adverse cardiovascular events, including heart attack and stroke, particularly in those who already have risk factors such as hypertension or diabetes. Regular monitoring of cardiovascular health is advisable during therapy.
Psychological Effects of Long-Term Use
Using Decapeptyl Depot for a time may lead to impacts, like feeling down or moody and experiencing changes, in mood swings or temperaments which can affect patients mentally and emotionally without them even realizing it earlier on in the treatment process thus monitoring their mental states is key as any warning signs of distress should be taken seriously with timely interventions to help them cope better with the effects of the medication offering them psychological support or counseling to aid in managing their emotional well being throughout the treatment period.
Recommendations for Concurrent Use with Other Therapies
When Decapeptyl Depot is used in combination with other therapies, such as chemotherapy or radiation, careful consideration should be given to drug interactions and cumulative effects. Coordination between the healthcare team is necessary to optimize treatment and minimize adverse effects.
Potential Impact on Fertility and Conception
Decapeptyl Depot can have a significant impact on fertility due to its suppression of sex hormones. Patients who are considering conception in the future should discuss alternative treatment options with their healthcare provider, as the effects on fertility may persist even after discontinuing the drug.
XV. Administration to Elderly Patients
Adjustments for Elderly Individuals Receiving Decapeptyl Depot
As individuals grow older and undergo treatment, their Decapeptyl Depot medication may need to be adjusted based on their age-related changes in functions such as hepatic systems that play a role in drug processing and removal from the body system—hence warranting close monitoring of organ functions and treatment outcomes.
Special Considerations for Prostate Cancer Treatment in Elderly
Prostate cancer treatment with Decapeptyl Depot in elderly individuals requires careful evaluation due to the increased risk of side effects, such as bone loss and cardiovascular complications. The benefits of treatment should be weighed against the potential risks, and regular monitoring should be prioritized.
Risks Associated with Osteoporosis and Bone Fractures
Elderly patients are at a heightened risk for osteoporosis and bone fractures due to long-term Decapeptyl Depot use. Bone density monitoring, along with preventive measures such as calcium and vitamin D supplementation, is crucial in managing this risk in older patients.
Monitoring for Signs of Adverse Reactions in Elderly Populations
Elderly patients should be closely observed for signs of adverse reactions, such as fatigue, mood changes, and cardiovascular symptoms, as these may be more pronounced in older adults. Regular follow-up appointments and prompt symptom reporting are essential for safely managing treatment in this age group.
XVI. Administration to Pregnant Women and Nursing Mothers
Contraindications During Pregnancy
Decapeptyl Depot is contraindicated during pregnancy due to its potential to disrupt hormonal regulation and affect fetal development. Use of this medication during pregnancy may cause harm to the fetus, and alternative treatment options should be explored.
Risk to Fetus and Potential Birth Defects
Exposure to Decapeptyl Depot during pregnancy may lead to serious birth defects, as the drug interferes with the body's hormonal balance. Pregnant women should avoid using Decapeptyl Depot and seek alternative therapeutic options that are safe during pregnancy.
Effect on Milk Production and Infant Health
Decapeptyl Depot may affect milk production and, therefore, should not be used by nursing mothers. The drug may also be excreted into breast milk, potentially impacting the infant's health and development. Nursing mothers should consult their healthcare provider for appropriate alternatives.
Alternative Treatments During Pregnancy and Lactation
Throughout pregnancy and while breastfeeding a baby, healthcare professionals need to explore alternative treatment approaches that are deemed safe for both the mother and the child's well-being. It may be more suitable to opt for hormonal treatments or those with a proven track record of safety during these stages to address medical conditions that necessitate intervention.
XVII. Administration to Children
Decapeptyl Depot for Precocious Puberty
Decapeptyl Depot is commonly used to treat precocious puberty in children. By suppressing the early onset of puberty, it allows for normal growth and development. The drug is effective in delaying puberty in children, especially those with idiopathic or central precocious puberty.
Age-Related Considerations for Pediatric Use
The use of Decapeptyl Depot in children requires careful consideration of age, weight, and overall health. Dosing should be adjusted based on these factors, and the potential long-term effects of hormonal suppression should be monitored closely. Pediatricians should assess the suitability of Decapeptyl Depot based on the child's condition.
Dosage Recommendations for Children and Adolescents
The dosing regimen for children and adolescents varies based on the underlying condition, such as precocious puberty. In general, treatment should begin with a lower dose and adjusted gradually. Specific dosing guidelines should be followed closely to ensure efficacy and minimize adverse effects.
Safety and Monitoring During Long-Term Use in Children
Continuous observation of children using Decapeptyl Depot for a period is essential to evaluate its impact, on their growth trajectory and bone strength over time. Regular medical check ups play a role in confirming that the hormonal suppression does not impede the childs physical and mental progress.
XVIII. Overdose and What to Do
Symptoms of Decapeptyl Depot Overdose
Overdosing on Decapeptyl Depot can lead to excessive hormone suppression, which may manifest as severe fatigue, dizziness, or imbalances in electrolytes. Symptoms may vary depending on the degree of overdose and the individual's health status.
Immediate Actions to Take in Case of Overdose
In case of an overdose occurrence it is crucial to seek assistance. Patients must promptly seek emergency treatment should they encounter symptoms. The primary focus of care will be, on normalizing hormone levels. Managing any acute symptoms arising from the overdose.
Medical Interventions and Management Options
Management of Decapeptyl Depot overdose involves stabilizing the patient and monitoring hormone levels. Specific interventions may include hormone replacement therapy or symptomatic treatment to address electrolyte imbalances and other complications.
Prevention of Overdose Through Proper Dosing
To prevent overdose, strict adherence to dosing guidelines is crucial. Healthcare providers should ensure that patients receive the correct dose based on their condition and regularly monitor therapy to avoid complications.
XIX. Handling and Storage of Decapeptyl Depot
Proper Storage Conditions for Decapeptyl Depot
Store Decapeptyl Depot, in an dry place where it is shielded from sunlight to maintain its effectiveness and safety, for use; avoid freezing it as this could compromise the drugs potency.
How to Store Unopened Vials and After Reconstitution
Unopened vials of Decapeptyl Depot should be kept in their original packaging, in a refrigerator between 2°C to 8°C (36°F to 46°F). Once reconstituted, the product should be used immediately or stored under specific conditions as instructed by the manufacturer.
Shelf Life and Expiration Date of the Product
Decapeptyl Depot comes with a specified shelf life, typically indicated on the packaging. The expiration date should be strictly adhered to, and expired medications should be discarded safely.
Safe Disposal Practices for Used Syringes and Vials
Used syringes and vials should be disposed of in accordance with local regulations for hazardous materials. Patients should never dispose of used injection equipment in household trash to avoid accidental needle-stick injuries. Safe disposal ensures environmental safety and prevents misuse.
Decapeptyl Depot FAQ
- What is Decapeptyl depot used for?
- What does Decapeptyl injection do?
- What is the use of Decapeptyl injection for pregnancy?
- What are the side effects of Decapeptyl 3.75 mg injection?
- What happens after Decapeptyl?
- What are the risks of taking Decapeptyl?
- Is Decapeptyl a chemotherapy?
- How long can I take Decapeptyl for?
- Can I get pregnant after Decapeptyl?
- Does Decapeptyl mature eggs?
- What is the use of Decapeptyl Depot Injection?
- Does Decapeptyl stop ovulation?
- Is it safe to take Decapeptyl while pregnant?
- Is it normal to bleed on Decapeptyl?
- What are the contraindications for Decapeptyl?
- How do you take Decapeptyl?
- What is the action of Decapeptyl?
- What is the route of Decapeptyl?
- Triptorelin where to inject?
- What is triptorelin injection?
- What is triptorelin used for in IVF?
- What is triptorelin medication used for?
- What is triptorelin used for?
- Triptorelin what does it do?
- Triptorelin how long?
- Triptorelin how does it work?
- Triptorelin how to use?
- Triptorelin how to give?
- How triptorelin works?
What is Decapeptyl depot used for?
Decapeptyl Depot Injection is prescribed for the treatment of prostate cancer in males and endometriosis in females for managing precocious puberty (early onset of puberty).
What does Decapeptyl injection do?
Decapeptyl helps reduce the production of estrogen by the ovaries.
What is the use of Decapeptyl injection for pregnancy?
In assisted reproductive technology (ART), this method is employed to lower the chances of cycle cancellation.
What are the side effects of Decapeptyl 3.75 mg injection?
The typical adverse reactions of Decapeptyl 3.75mg Injection may encompass flushes, decreased libido, testicular atrophy, breast soreness or enlargement headedness, migraines, flu-like symptoms, queasiness, retching, bowel issues, and swelling in the lower limbs.
What happens after Decapeptyl?
The impact of Decapeptyl often stems from estrogen levels, leading to symptoms akin to those experienced during menopause, causing side effects that affect most women, ranging from mild to severe in intensity, typically manifesting as hot flashes and night sweats.
What are the risks of taking Decapeptyl?
The likelihood of experiencing heart rhythm issues could potentially rise.
Is Decapeptyl a chemotherapy?
hormonal therapy drug
How long can I take Decapeptyl for?
You shouldn't require treatment for more than half a year.
Can I get pregnant after Decapeptyl?
Yes
Does Decapeptyl mature eggs?
Using it to avoid ovarian hyperstimulation syndrome (OHSS), a common approach is employed to trigger egg maturation and ovulation following a protocol involving GnRH antagonists in the past.
What is the use of Decapeptyl Depot Injection?
Treatment of prostate cancer
Does Decapeptyl stop ovulation?
Decapeptyl® is utilized in these procedures to inhibit the body's natural LH production and prevent ovulation from occurring.
Is it safe to take Decapeptyl while pregnant?
Before beginning Decapeptyl treatment, it is important to confirm the absence of pregnancy through testing.
Is it normal to bleed on Decapeptyl?
It's pretty common to experience bleeding in the month of starting the treatment.
What are the contraindications for Decapeptyl?
Avoid using Decapeptyl if you have a sensitivity to triptorelin or any of its components hypersensitivity to gonadotropin-releasing hormone (like GnRH) and are pregnant or breastfeeding. If you are a woman who could become pregnant. Make sure to get checked before starting treatment to rule out pregnancy.
How do you take Decapeptyl?
Decapeptyl is administered through an injection, which means it is injected under the skin in the abdominal region after cleaning the area with an alcohol swab.
What is the action of Decapeptyl?
Triptorelin, also known by the brand name Decapeptyl, is a medication that functions as an analog of gonadotropin-releasing hormone. It works by suppressing the production of both LH (luteinizing hormone) and FSH (follicle stimulation hormone).
What is the route of Decapeptyl?
Intramuscular
Triptorelin where to inject?
muscle of either buttock
What is triptorelin injection?
Triptorelin is used as a GnRH agonist to help with managing advanced prostate cancer symptoms.
What is triptorelin used for in IVF?
It leads to a drop in FSH and LH levels along with a decline in the production of hormones from the ovaries and testes, typically occurring 2 to 4 weeks after starting treatment.
What is triptorelin medication used for?
to treat advanced prostate cancer.
What is triptorelin used for?
Triptorelin is commonly prescribed for managing late-stage prostate cancer in patients.
Triptorelin what does it do?
Triptorelin, a gonadotropin-releasing hormone (GnRH), acts as an inhibitor of testosterone production in men and estrogen production in women and is commonly employed in the treatment of advanced prostate cancer.
Triptorelin how long?
Take 11 milligrams once every three months starting within the five days of your cycle and continue for a maximum of six months.
Triptorelin how does it work?
Triptorelin is a medication for therapy that functions by imitating the actions of gonadotropin-releasing hormone (known as GnRH), prompting the gland to produce both Luteinizing Hormone (abbreviated as LH) and Folicle stimulation Hormone (often referred to as FSH).
Triptorelin how to use?
Administer 4 weekly injections of 1 milligram into the buttocks using an injection method.
Triptorelin how to give?
Buttocks
How triptorelin works?
The start may initially stimulate the release of gonadotropins. Eventually, this decreases their production and release, leading to reduced testosterone and estrogen synthesis.