Pantpas IV Injection
Introduction
Pantpas IV Injection is a potent pharmaceutical solution designed for addressing a variety of gastrointestinal conditions. Classified under the proton pump inhibitor (PPI) category, it is pivotal in managing conditions characterized by excessive stomach acid production. Recognized for its therapeutic efficacy, this medication is widely used in clinical settings to treat both acute and chronic conditions.
Manufactured by leading pharmaceutical companies, Pantpas IV Injection is readily available in medical stores and hospitals worldwide, ensuring consistent patient access and care.
Composition
The primary active ingredient in Pantpas IV Injection is Pantoprazole Sodium, a highly effective agent in reducing gastric acid secretion. Complementing this are various inactive ingredients and excipients, which ensure stability, efficacy, and patient tolerability. The formulation is typically presented as a lyophilized powder that requires reconstitution before administration.
Each component works synergistically to deliver the intended therapeutic effects while maintaining the medication's shelf stability and safety profile.
Pantoprazole sodium sesquihydrate
This medication is part of a category known as proton pump inhibitors (PPIs). Its function involves reducing the production of stomach acid to alleviate symptoms and promote the healing process.
Pantoprazole sodium vs omeprazole
Some omeprazole can be bought over the counter for managing heartburn in adults; however, pantoprazole requires a prescription.
Uses
Pantpas IV Injection is utilized for a wide range of conditions, including:
- Treatment of gastroesophageal reflux disease (GERD), mitigating heartburn and acid regurgitation.
- Management of Zollinger-Ellison syndrome, characterized by excessive gastric acid production.
- Healing of erosive esophagitis caused by chronic acid reflux.
- Prevention of gastric ulcers induced by long-term NSAID use.
- Combination therapy for Helicobacter pylori eradication, enhancing treatment outcomes.
- Other approved indications such as peptic ulcer disease and dyspepsia.
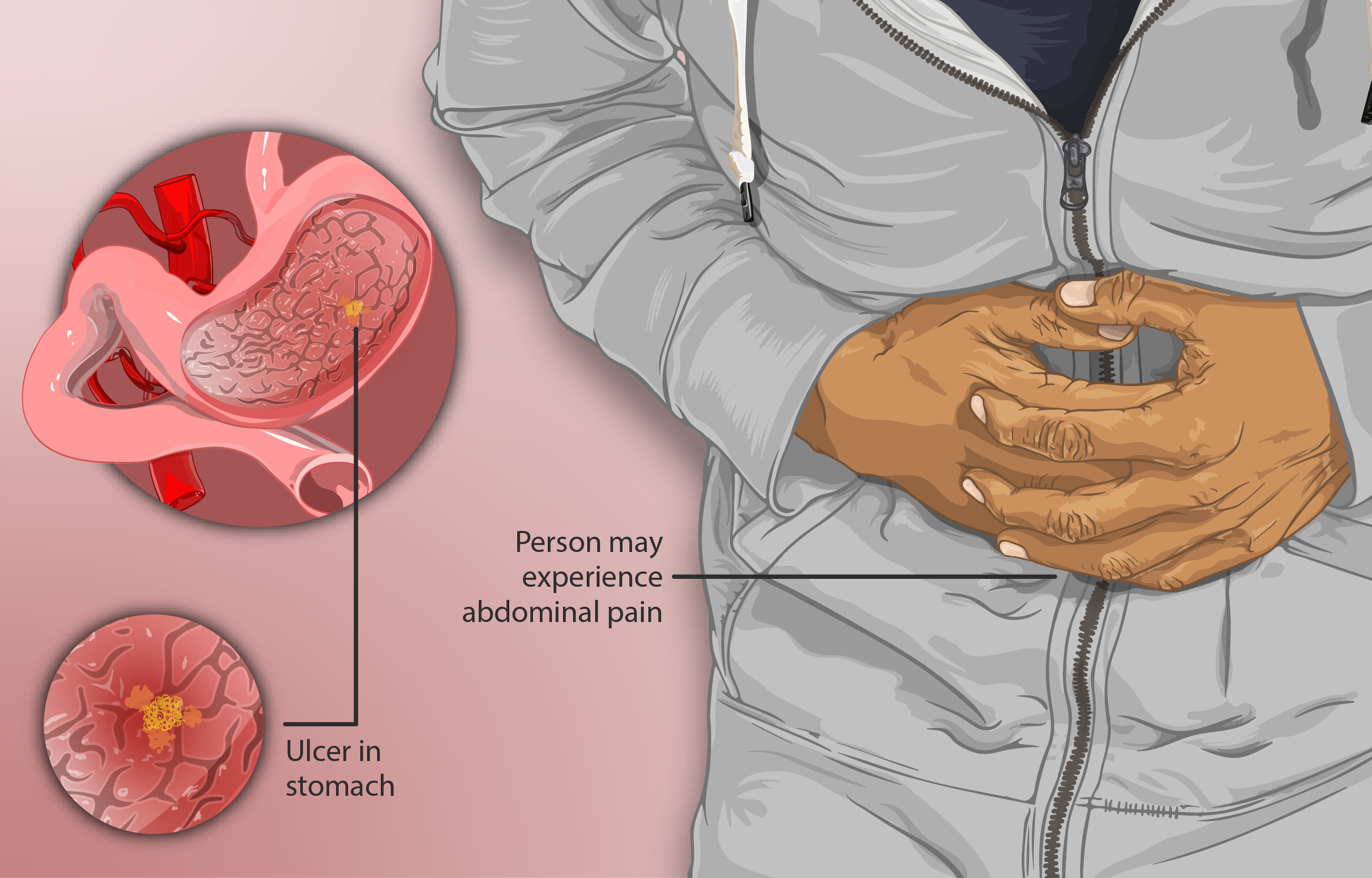
Off-label Uses
Pantpas IV Injection is also employed in several off-label applications:
- Management of stress-related mucosal damage in critically ill patients.
- Control of bleeding peptic ulcers to stabilize patients before further intervention.
- Reduction of acid aspiration risk during surgical anesthesia.
- Other non-standard but clinically beneficial uses observed in practice.

How it Works
Pantoprazole operates by selectively inhibiting the H+/K+ ATPase enzyme system in the gastric parietal cells. This blockade significantly reduces the secretion of gastric acid, providing relief from hyperacidity-related conditions. Its pharmacodynamic effect stabilizes the gastric mucosal barrier, aiding in the healing of acid-induced tissue damage.
As a proton pump inhibitor, Pantoprazole's impact extends beyond symptomatic relief, contributing to long-term management of acid-related disorders.
Dosage and Administration
The dosage of Pantpas IV Injection varies based on the medical condition:
- GERD: 40 mg once daily, typically for a duration of 7-10 days.
- Zollinger-Ellison syndrome: Higher doses administered under medical supervision.
- Erosive esophagitis: Standard 40 mg dose for short-term use.
The injection must be reconstituted and diluted according to the manufacturer's instructions. It is administered via IV infusion over 15-30 minutes, ensuring patient safety and optimal drug efficacy.
Storage
Make sure to keep Pantpas IV Injection in a dry place, from sunlight and moisture, for proper storage precautions. After mixing the solution as instructed, refer to the recommended time frame for usage to maintain its effectiveness. Get rid of any portions correctly to prevent contamination risks. Storing it properly is crucial to keep the medication stable and effective throughout its shelf life.
Side Effects
Pantpas IV Injection is usually well received by patients. There can be instances where some individuals may encounter reactions.
Common:
Nausea, vomiting, diarrhea, constipation, abdominal discomfort, and flatulence.
Rare but severe:
Hypomagnesemia, Clostridium difficile infection, hepatotoxicity, and jaundice.
Patients experiencing severe or persistent side effects should seek immediate medical attention.
Important Precautions
Ensuring patient safety while administering Pantoprazole requires adherence to critical precautions. Regular monitoring of serum magnesium levels is essential, particularly for patients undergoing prolonged treatment, as hypomagnesemia can have serious consequences.
Long-term use of proton pump inhibitors, including Pantoprazole, has been associated with an increased risk of bone fractures, especially in the hip, wrist, and spine. This necessitates additional caution in patients with underlying osteoporosis or those on high doses.
Patients with a documented history of hypersensitivity to PPIs should avoid this medication to prevent allergic reactions. Individuals with conditions such as severe osteoporosis or liver disease require tailored treatment plans to mitigate potential risks.
Pantoprazole sodium nursing considerations
When taking Pantoprazole medication, be aware of side effects, like headaches and stomach issues, such as diarrhea and abdominal discomfort, along with feelings of nausea. Remember to keep an eye on the patient for these signs and inform the doctor promptly if any negative reactions occur.
Contraindications
Pantoprazole should not be used in patients with a known hypersensitivity to the drug or other proton pump inhibitors. Severe hepatic impairment or cirrhosis poses a significant contraindication due to the risk of exacerbating liver dysfunction.
Pregnancy category considerations also limit the use of this drug in certain populations. The risks and benefits must be carefully weighed in pregnant women and other vulnerable groups to ensure optimal outcomes.
Drug Interactions
Drug interactions with Pantoprazole can alter the efficacy of co-administered medications. For example:
- Antiretroviral drugs such as atazanavir may lose efficacy when used with Pantoprazole due to pH-dependent absorption.
- Medications like ketoconazole and itraconazole, which require an acidic environment for absorption, may show reduced effectiveness.
- Interactions with anticoagulants such as warfarin could influence coagulation parameters, necessitating close monitoring.
- Potentially synergistic or antagonistic effects with other drugs require careful evaluation before concurrent use.
Administration in Special Populations
Elderly Patients
As people grow older, they may require dosage adjustments for medications due to changes in how their bodies process drugs over time. Regular monitoring can help reduce the risks linked to changes in drug metabolism and clearance as individuals age.

Pregnant Women and Nursing Mothers
While Pantoprazole is generally considered safe for use during pregnancy in cases with a safety record if needed; it is advisable to limit its use to when absolutely necessary under such circumstances since breastfeeding mothers should take into account the potential effects on their infants due, to the possibility of trace amounts being passed through breast milk.

Children
Children can be prescribed Pantoprazole based on guidelines to ensure their safety and receive effective treatment that caters to their specific needs depending on their age and weight requirements.
Overdose
Symptoms of Pantoprazole overdose include nausea, confusion, headache, and dizziness. Immediate intervention is crucial to mitigate adverse effects. Supportive measures such as gastric lavage or administration of activated charcoal can be employed to reduce drug absorption.
While specific antidotes for Pantoprazole overdose are unavailable, symptomatic management and close monitoring can ensure patient recovery.
Handling Precautions
Proper handling of Pantoprazole is paramount to ensure efficacy and safety:
- Reconstitution and dilution should follow the manufacturer's guidelines precisely.
- Contamination during preparation must be avoided to maintain sterility.
- Healthcare professionals should adhere to best practices for safe administration, minimizing risks for both the patient and themselves.
Warnings
Abrupt discontinuation of Pantoprazole can lead to rebound acid hypersecretion. Patients should be gradually weaned off under medical supervision. Long-term use carries the potential for dependency and may mask underlying gastric malignancies, warranting vigilant monitoring.
Special care is needed when administering the drug to individuals with compromised immunity, as they may be more susceptible to adverse effects.

Careful Administration
Patients who have issues with their kidneys or liver may need to adjust their dosages to avoid buildup and harmful effects in their bodies.It is important to customize treatment plans for patients who're very ill or have weakened systems to ensure safety and positive treatment results. People with health conditions need care that includes incorporating Pantoprazole into their overall treatment plan while taking into account possible interactions and restrictions.
Pantpas IV Injection FAQ
- What is a pantoprazole IV injection used for?
- How long does IV pantoprazole take to work?
- Can pantoprazole be given intravenously?
- What organs does pantoprazole affect?
- What is the main side effect of pantoprazole?
- How fast do you push IV pantoprazole?
- How often can you give IV pantoprazole?
- Why are patients given pantoprazole?
- Can I take pantoprazole at night instead of morning?
- Is pantoprazole safe for kidneys?
- How to use pantoprazole injection?
- When should I avoid pantoprazole?
- Does pantoprazole need to be refrigerated?
- What happens if you give pantoprazole too fast?
- What not to mix with pantoprazole?
- Can I take vitamins while taking pantoprazole?
- What organ does pantoprazole affect?
- What happens if I eat right after taking pantoprazole?
- What is a safer alternative to pantoprazole?
- What medicines can you not take with pantoprazole?
- Can I take amlodipine and pantoprazole together?
- Can I take antibiotics and pantoprazole at the same time?
- Can I eat bananas while taking pantoprazole?
- How long does it take pantoprazole to heal acid reflux?
- Does pantoprazole cause weight gain?
- Is pantoprazole over the counter?
- Is pantoprazole the same as omeprazole?
- Can I take pantoprazole at night after dinner?
- Does pantoprazole cause constipation?
- Why take pantoprazole first thing in the morning?
What is a pantoprazole IV injection used for?
Injectable pantoprazole is prescribed for cases where there is an excess of stomach acid present.
How long does IV pantoprazole take to work?
15–60 minutes
Can pantoprazole be given intravenously?
In trials, intravenous pantoprazole has been proven to be both safe and effective.
What organs does pantoprazole affect?
liver, kidneys, and heart
What is the main side effect of pantoprazole?
Headaches and diarrhea
How fast do you push IV pantoprazole?
15 minutes at a rate of approximately 7 mL/min
How often can you give IV pantoprazole?
Every 12 hours
Why are patients given pantoprazole?
People often take Pantoprazole to address situations where the stomach produces an excess of acid, such as treating esophagitis or the discomfort known as "heartburn " triggered by gastroesophageal reflux disease (GERD)—a condition in which stomach acid flows back into the esophagus.
Can I take pantoprazole at night instead of morning?
Usually, Pantoprazole is taken daily either on a stomach or with food later on in the evening; however, you can also take it twice a day. Once in the morning and again, in the evening. For optimal effects that start to show within two to three days of consistent use.
Is pantoprazole safe for kidneys?
The average time of taking pantoprazole before experiencing acute kidney injury is three months; however, it has also been noted to happen after taking pantoprazole.
How to use pantoprazole injection?
The suggested dose for adults is 80 mg, which is given through an IV every 12 hours.
When should I avoid pantoprazole?
Caution about fundic gland polyps: Prolonged e d use of pantoprazole ( exceeding 1 year) may lead to the development of fundic gland polyps – growths forming in the stomach lining that could potentially progress into growths over time. To reduce the risk of these polyps forming it is advisable to limit the duration of pantoprazole use as possible.
Does pantoprazole need to be refrigerated?
Storing Pantoprazole sodium diluted in saline at concentrations of 0 0 mg/mL or 0 0 mg/mL in bags is safe for up to 3 days at room temperature when exposed to light or up to 28 days when refrigerated and protected from light.
What happens if you give pantoprazole too fast?
When pantoprazole is infused at rates, it results in a decrease in the end systolic LV pressure by lowering the heart rate, myocardial contractility, and arterial elastance.
What not to mix with pantoprazole?
- afatinib
- atazanavir
- dasatinib
- delavirdine
- edoxaban
- indinavir
- itraconazole.
Can I take vitamins while taking pantoprazole?
If you lack iron or suffer from anemia, please consult with your physician before taking a combination of multivitamins and prenatal vitamins along with pantoprazole medication .
What organ does pantoprazole affect?
Stomach
What happens if I eat right after taking pantoprazole?
You can consume food and beverages as usual while using pantoprazole.
What is a safer alternative to pantoprazole?
- Omeprazole (Prilosec)
- Esomeprazole (Nexium)
- Lansoprazole (Prevacid)
- Dexlansoprazole (Dexilant)
- Rabeprazole (Aciphex)
What medicines can you not take with pantoprazole?
Avoid combining pantoprazole with medications that include rilpivirine.
Can I take amlodipine and pantoprazole together?
When Amlodipine is taken together with Pantoprazole, it may lead to a reduction in the metabolism of the latter.
Can I take antibiotics and pantoprazole at the same time?
Patients should be careful when using Pantoprazole if they are taking medications that rely on the acidity of the stomach for absorption, like antibiotics and iron supplements.
Can I eat bananas while taking pantoprazole?
Yes
How long does it take pantoprazole to heal acid reflux?
It usually takes a few days for pantoprazole to begin showing its effects on conditions; in some instances, you might need to keep taking it for several months or even require treatment for the rest of your life.
Does pantoprazole cause weight gain?
You might experience an increase in weight while using tablets.
Is pantoprazole over the counter?
No
Is pantoprazole the same as omeprazole?
NO
Can I take pantoprazole at night after dinner?
Yes
Does pantoprazole cause constipation?
Yes
Why take pantoprazole first thing in the morning?
It assists in stopping the production of stomach acid that could result in reflux.