Prazivet Cat
- I. Introduction
- II. Composition and Formulation
- III. Mechanism of Action: How Prazivet Cat Works
- IV. Approved Uses and Indications
- V. Off-Label Uses and Alternative Applications
- VI. Dosage and Administration Guidelines
- VII. Side Effects and Adverse Reactions
- VIII. Contraindications, Warnings, and Precautions
- IX. Special Administration Considerations
- X. Handling, Storage, and Overdose Management
- XI. Drug Interactions and Comprehensive Safety Measures
I. Introduction
A. Overview of Prazivet Cat
Prazivet is a dewormer that targets a variety of parasites found in cats. It is designed to address infections that involve both roundworm and tapeworm.

B. Significance in Feline Veterinary Care
The formulation stands as a pivotal element in contemporary feline medicine. It addresses an array of conditions with precision. Its use is marked by:
- Effective symptomatic alleviation
- Enhanced immunomodulatory support
- Reduction of inflammatory sequelae
II. Composition and Formulation
A. Active Ingredients and Their Roles
The formulation integrates a series of bioactive constituents that synergize to achieve a therapeutic milieu. These active ingredients are meticulously selected for their ability to:
- Praziquantel: Targets tapeworms.
- Pyrantel Pamoate: Targets roundworms and hookworms.
B. Inactive Ingredients and Excipients
Inactive ingredients serve as the silent custodians of stability and bioavailability. They include:
- Stabilizers
- Preservatives
- Solubilizers
Although inert, these excipients are indispensable in facilitating uniform distribution and efficacy of the active moieties.
Fenbendazole vs pyrantel pamoate
In the field of medicine, fenbendazole is a choice for treating various parasitic infections in livestock, pets, and even animals in zoos. It is especially effective against worms like roundworms, hookworms, and whipworms. On the other hand, pyrantel pamoate is frequently prescribed for dogs, cats, and horses to combat intestinal parasites such as roundworms and pinworms.

III. Mechanism of Action: How Prazivet Cat Works
A. Pharmacodynamics and Therapeutic Effects
The therapeutic effects of Prazivet Cat are achieved by influencing pathways in a way that involves adjusting receptor binding and interfering with signal transduction while promoting tissue repair processes effectively and efficiently.
B. Pharmacokinetics in Felines
The pharmacokinetic profile of Prazivet Cat in felines is characterized by rapid absorption, targeted tissue distribution, and efficient clearance. This is crucial for achieving therapeutic plasma concentrations while minimizing systemic exposure. Key points include:
- Swift systemic absorption
- Controlled metabolism
- Efficient excretion pathways
C. Comparison with Similar Veterinary Medications
Compared to analogous veterinary products, Prazivet Cat distinguishes itself with superior efficacy and a commendable safety profile. It offers:
- Extended duration of action
- Reduced incidence of adverse effects
- Enhanced receptor specificity
Its distinct pharmacological attributes render it a preferred option in contemporary feline therapeutics.
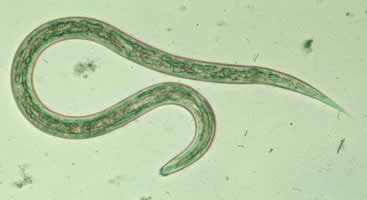
IV. Approved Uses and Indications
Treatment and control of gastrointestinal parasites in cats, including:
- Tapeworms (e.g., Dipylidium caninum, Taenia taeniaeformis, Echinococcus multilocularis).
- Roundworms (e.g., Toxocara cati, Toxascaris leonina).
- Hookworms.
V. Off-Label Uses and Alternative Applications
A. Common Off-Label Uses in Feline Medicine
Beyond its primary indications, Prazivet Cat is occasionally employed off-label to harness its ancillary therapeutic benefits. Veterinary practitioners have observed its utility in:
- Adjunctive therapy for chronic conditions
- Support in neoplastic management
- Adjunctive anti-inflammatory interventions
These applications, while not formally approved, are predicated on emerging clinical insights and practitioner discretion.
VI. Dosage and Administration Guidelines
A. Recommended Dosage by Weight and Condition
Dosage regimens are meticulously calibrated to accommodate variances in feline weight and the specific pathological condition at hand. Precision is paramount. Key considerations include:
- Weight-based dosing adjustments: Dosage: 1 tablet per 10 kg (22 lbs.) bodyweight.
- Condition-specific titration
- Maintenance of therapeutic plasma levels
Accurate dosing ensures both efficacy and safety, minimizing the risk of underdosing or overdose.
B. Administration Routes and Techniques
Multiple administration routes are available, each tailored to optimize absorption and clinical effectiveness. Techniques include:
- Oral ingestion with precision measurement
- Injectable delivery under aseptic conditions
- Topical application for localized treatment
Each route is chosen based on the clinical scenario, ensuring maximal bioavailability and therapeutic benefit.
VII. Side Effects and Adverse Reactions
A. Overview of Potential Side Effects
Although Prazivet Cat is predominantly well-tolerated, certain side effects may manifest. Clinicians should be vigilant, as adverse reactions may include:
These effects are typically minor and self-limiting; however, prompt recognition remains essential.

B. Detailed Analysis of Common Side Effects
A closer scrutiny of the adverse reaction profile reveals that gastrointestinal upset and transient behavioral alterations are the most recurrent. The side effects are often characterized by:
- Mild nausea and sporadic vomiting
- Brief periods of drowsiness
Detailed monitoring facilitates timely intervention, thereby ensuring the sustained well-being of the feline patient.
VIII. Contraindications, Warnings, and Precautions
A. Identified Contraindications for Use
The application of this formulation is strictly contraindicated in felines with documented hypersensitivities. Conditions such as:
- Allergic manifestations to constituent compounds
- Pre-existing hepatic dysfunction
- Severe renal insufficiency
Vigilance is paramount; even subtle idiosyncratic responses warrant immediate discontinuation.

B. Critical Warnings and Risk Factors
The therapeutic regimen necessitates cautious appraisal of risk factors. Short-term use might precipitate:
- Exacerbation of latent infections
- Transient hemodynamic instabilities
- Potential for hypersensitive cascades
Long and intricate clinical observations must be undertaken to circumvent pernicious sequelae.
IX. Special Administration Considerations
A. Careful Administration Techniques and Best Practices
Precision in administration is critical to therapeutic success. The regimen mandates:
- Utilization of calibrated delivery systems
- Adherence to aseptic technique protocols
- Gradual titration to mitigate abrupt pharmacodynamic shifts
Both succinct instructions and elaborate procedural frameworks must be concurrently observed.
B. Administration in Elderly Felines
Geriatric felines require bespoke therapeutic modifications owing to their diminished metabolic clearance. Essential considerations include:
- Lower initial dosages with cautious titration
- Enhanced frequency of physiological monitoring
- Attentive observation for atypical adverse reactions
The intricate interplay of age-related pharmacokinetic variables demands a calibrated and judicious approach.
C. Guidance for Pregnant Women and Nursing Mothers in Feline Care
The intersection of maternal care and feline treatment necessitates a circumspect methodology. Recommendations incorporate:
- Conservative dosing to minimize fetal exposure
- Rigorous assessment of lactational transmission risks
- Enhanced vigilance during peripartum periods
A synergistic approach, balancing efficacy with safety, is imperative to safeguard both maternal and offspring well-being.

X. Handling, Storage, and Overdose Management
A. Proper Storage Conditions and Stability
Optimal storage conditions are vital for preserving the formulation's stability and potency. It is recommended to:
- Maintain a controlled temperature environment
- Avoid exposure to direct sunlight and humidity
- Utilize secure, tamper-evident packaging
Such meticulous storage protocols ensure the formulation's integrity over extended periods.
B. Handling Precautions and Safety Measures
Handling procedures must be executed with scrupulous care. Key safety measures include:
- Use of personal protective equipment
- Adherence to standardized handling protocols
- Immediate decontamination in the event of spillage
A synthesis of both pragmatic and esoteric safety protocols mitigates inadvertent exposure risks.

C. Recognizing and Managing Overdose Scenarios
Overdose events, while infrequent, necessitate rapid and informed intervention. Hallmarks of overdose include:
- Marked alteration in physiological parameters
- Exacerbated adverse reactions
- Signs of systemic toxicity
Prompt administration of supportive measures, coupled with intensive monitoring, is paramount to restoring homeostasis.
XI. Drug Interactions and Comprehensive Safety Measures
A. Overview of Potential Drug Interactions
The formulation's multifarious pharmacodynamics may intersect with other concomitantly administered agents. Notable interactions may arise with:
- Concurrent immunosuppressants
- Competing enzymatic substrates
- Medications with overlapping toxicity profiles
A thorough interrogation of the feline's medication history is indispensable.
B. Strategies to Mitigate Interaction Risks
Proactive stratagems must be implemented to obviate deleterious drug interactions. Recommended strategies encompass:
- Comprehensive review of concurrent therapies
- Utilization of staggered administration schedules
- Employing pharmacovigilance to monitor emergent interactions
Integrative risk management is essential to forestall adverse polypharmacy consequences.
C. Long-Term Safety Monitoring Recommendations
Sustained vigilance is imperative. Recommended measures include:
- Periodic biochemical and hematological assessments
- Longitudinal clinical evaluations
- Regular updates to the therapeutic protocol based on emerging evidence
This continuous surveillance ensures that long-term safety remains uncompromised.
Prazivet Cat FAQ
- What is prazivet used for?
- What does praziquantel treat in cats?
- What does praziquantel cure?
- Do cats poop out tapeworms after being dewormed?
- Is praziquantel safe for cats?
- What worms are killed by praziquantel?
- How quickly does praziquantel work?
- Can you mix praziquantel with water?
- What is pyrantel used for?
- How long does pyrantel take to deworm?
- Is pyrantel better than mebendazole?
- Is pyrantel a good dewormer?
- Which is better, pyrantel or albendazole?
- What worms does pyrantel not treat?
- Can you use pyrantel pamoate for cats?
- What is pyrantel pamoate used to treat?
- Is pyrantel effective for tapeworms in cats?
- How many days do you give pyrantel pamoate?
What is prazivet used for?
Dogs that are 3 weeks old and weigh over 1 lb can be given Prazivet (a combination of Praziquantel/Pyrantel Pamoate/Febantel) which is used to deworm them and can also help treat tapeworms as wel, as other parasites, like roundworms and hookworms.
What does praziquantel treat in cats?
Eliminate the tapeworm infections found in cats and kittens known as Dipylidium caninum and Taenia taeniaeformis.
What does praziquantel cure?
Treatment with praziquantel is commonly employed for addressing schistosomiasis – a condition referred to as snail fever or bilharzia characterized by an infection affecting the tract or intestines caused by schistosoma (blood fluke) a type of flatworm parasite.
Do cats poop out tapeworms after being dewormed?
Normally tapeworm infections get resolved inside a cats stomach; however if your furry companion has tapeworm infestations in their system you might observe a few of them being expelled in their excrement.
Is praziquantel safe for cats?
It is completely safe to use in cats at a dosage of 40 mg/kg.
What worms are killed by praziquantel?
To combat schistosoma (a worm that resides in the bloodstream) and liver fluke (a worm found near or in the liver) praziquantel is administered as a medication belonging to the class effectively eliminating the worms from the system.
How quickly does praziquantel work?
1-2 hours
Can you mix praziquantel with water?
To effectively combat flukes and tapeworm infestations it's important to dissolve praziquantel in water before administering it to the organisms.
What is pyrantel used for?
People often use this medication to treat worm infections such, as pinworms and roundworms or hookworms are cases treated with this drug known as Pyrantel which belongs to a class of medicines referred to as anthelmintics that work by immobilizing the worms through paralysis so that the body can expel them through stool excretion more easily.
How long does pyrantel take to deworm?
1-2 hrs
Is pyrantel better than mebendazole?
Both drugs have proven to be effective in the treatment of pinworm infection.
Is pyrantel a good dewormer?
Many people consider Pyrantel to be a cost remedy for eliminating parasites since a dose is frequently sufficient to treat the infection.
Which is better, pyrantel or albendazole?
Albenzadole is known for its success rate of 72% while Pyrantel Pamoate has an efficacy rate of 32%.
What worms does pyrantel not treat?
Doctors commonly recommend Pyrantel pamoate for treating worm infestations such as hookworm and Ascaris infections but its efficacy might be limited in treating trichuriasis.
Can you use pyrantel pamoate for cats?
It's highly reliable and efficient for addressing roundworms and hookworms in felines.
What is pyrantel pamoate used to treat?
This medicine is commonly used for the treatment of worm infections, like pinworms and hookworms.
Is pyrantel effective for tapeworms in cats?
Cats are given Pyrantel to deal with roundworm and hookworm infections. However, it doesn't work against whipworm or tapeworm infestations.
How many days do you give pyrantel pamoate?
2 weeks for pinworm infections