Rituximab
- I. Introduction
- II. What is Rituximab? How it Works
- III. Indications: Approved Uses of Rituximab
- IV. Off-Label Uses of Rituximab
- V. Dosage and Administration
- VI. Contraindications and Warnings
- VII. Side Effects and Adverse Reactions
- VIII. Special Precautions and Careful Administration
- IX. Drug Interactions and Compatibility
- X. Administration to Special Populations
- XI. Overdose Management and Handling Precautions
- XII. Conclusion
I. Introduction
Brief Overview of Rituximab
Rituximab, commonly known as Rituxan is a type of monoclonal antibody that is specifically created to target and remove cells within the immune system. Its primary focus is, on CD20, which is a protein found on the outer surface of B lymphocytes.
Historical Development and FDA Approval
This medication of origin was first approved by the FDA in 1997, and its usage has since expanded. While it was initially authorized for the treatment of Non-Hodgkins Lymphoma further clinical trials have paved the way, for therapeutic applications.
Importance in Modern Medicine
A groundbreaking approach to treatment. Functions as a medication alongside other therapies. Reduces reliance on doses of corticosteroids.
II. What is Rituximab? How it Works
Basic Composition of Rituximab
Rituximab is an antibody that combines both mouse and human monoclonal antibodies. It is made up of a mixture of human and mouse immunoglobulins IgG1 κ with the aim to utilize the best qualities from both species.
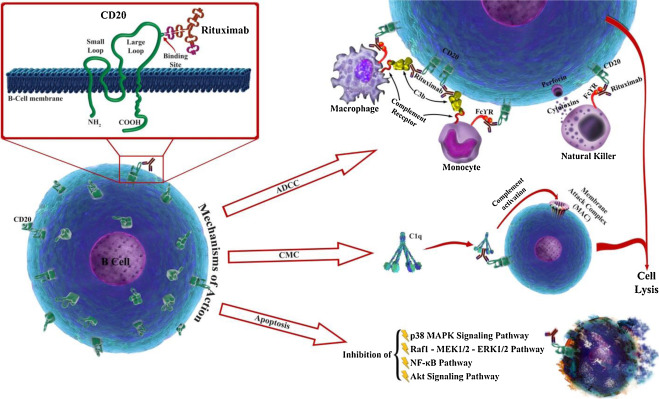
Mechanism of Action: Targeting CD20 on B Cells
Rituximab helps eliminate these B cells by attaching to CD20 proteins, a process called antibody cellular cytotoxicity. This can be highly effective when B cells are overactive or cancerous.

Pharmacokinetics and Pharmacodynamics
The duration of action for Rituximab varies between 32 and 49 hours depending on the medical condition it is being used to treat. One of its effects involves reducing the number of B cells present in both the bloodstream and tissues.
III. Indications: Approved Uses of Rituximab
Rheumatoid Arthritis
Rituximab is a monoclonal antibody that targets the CD20 antigen and is used as a therapeutic biologic agent in rheumatoid arthritis (RA) and other autoimmune disorders and lymphoproliferative disorders 12. It is approved for RA patients who do not respond adequately to disease-modifying antirheumatic drugs 3. Rituximab has been shown to be effective in managing moderate to severe cases of RA, often used alongside methotrexate 3.
3: UpToDate 1: SpringerLink 2: UpToDate
Non-Hodgkin's Lymphoma
Rituximab is a monoclonal antibody that targets the CD20 antigen and is used as a therapeutic biologic agent in the treatment of different types of cancer, including non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and Waldenström’s macroglobulinemia 12. It was the first therapeutic antibody approved for oncology patients and was the top-selling oncology drug for nearly a decade with sales reaching $8.58 billion in 2016 3. Rituximab is effective in treating B-cell malignancies, including diffuse large B-cell lymphoma, follicular lymphoma, and chronic lymphocytic leukemia 3.
3: Frontiers 1: NCI 2: Cancer Research UK
Chronic Lymphocytic Leukemia
- Utilized in later stages.
- Can be employed as a monotherapy or part of a combination regimen.
Other FDA-Approved Conditions
Rituximab is a monoclonal antibody that targets the CD20 antigen and is used as a therapeutic biologic agent in the treatment of different types of cancer, including non-Hodgkin’s lymphoma, chronic lymphocytic leukemia, and Waldenström’s macroglobulinemia 1 . It was the first therapeutic antibody approved for oncology patients and was the top-selling oncology drug for nearly a decade with sales reaching $8.58 billion in 2016 2. Rituximab has also been approved for treating Granulomatosis with Polyangiitis (GPA) and Microscopic Polyangiitis (MPA), which are related systemic vasculitides that, along with eosinophilic granulomatosis with polyangiitis (Churg-Strauss), make up the antineutrophil cytoplasmic autoantibody (ANCA)-associated vasculitides 3 .
2: Frontiers 1: NCI 3: UpToDate : Cancer Research UK : UpToDate
IV. Off-Label Uses of Rituximab
Multiple Sclerosis
Rituximab is a therapy that depletes B-cells, which are involved in the immune attack on the nervous system in multiple sclerosis (MS). Rituximab is not approved as a treatment for MS, but it is commonly used off-label to reduce relapse risk and delay disability progression in MS patients 1. A study indicated its effectiveness and safety for up to seven years 1.
1: Multiple Sclerosis News Today
Lupus
Rituximab is a monoclonal antibody that targets B-cells and has shown efficacy in treating Systemic Lupus Erythematosus (SLE) 1. However, limited evidence supports its effectiveness in treating SLE 2. A study suggests that the combination of rituximab and belimumab may be more effective than rituximab alone 2.
2: Trials 1: Rheumatology
Autoimmune Hemolytic Anemia
- Usage in pediatric cases.
- Short-term remissions noted.
Ethical and Regulatory Concerns of Off-Label Use
Using Rituximab for purposes not approved by authorities raises ethical and legal concerns, which require thorough clinical justification.
V. Dosage and Administration
Standard Dosage Guidelines
The recommended dosage usually depends on a person's weight and the specific condition being treated. Typically, the medication is. Given through an intravenous injection.
Route of Administration: Intravenous and Subcutaneous
Mostly given through the veins, administering it under the skin is also an option.
Timing and Frequency of Dosing
The treatment plan often includes rounds, with each round requiring one or more infusions.
Adjustments for Special Populations (Elderly, Renal Failure, etc.)
Patients with kidney or liver problems may need to adjust their medication doses. Similarly, older adults may require more careful monitoring.
VI. Contraindications and Warnings
Absolute Contraindications
People with a heightened sensitivity to Rituximab or any of the ingredients, in this medication, should avoid using it.
Relative Contraindications
- Active infection.
- History of cardiac arrhythmia.
Boxed Warnings and Special Alerts
The FDA has released warnings regarding the possibility of life-threatening reactions during infusions, skin and mucous membrane reactions, as well as reactivation of Hepatitis B.
VII. Side Effects and Adverse Reactions
Common Side Effects: Infusion Reactions, Fatigue, Nausea
Mild to moderate infusion reactions are pretty standard. They can typically be handled by taking medication beforehand and administering the infusion more slowly.
Serious Side Effects: Cardiac Events, Infections
Serious heart problems and severe infections, although uncommon, require medical attention.
Long-Term Adverse Effects
Extended use of a product increases the likelihood of weakening the system and, in turn, makes individuals more susceptible to opportunistic infections.
VIII. Special Precautions and Careful Administration
Monitoring Blood Counts
Regular blood tests are essential for identifying conditions like neutropenia or any abnormalities in the blood cells.
Hepatitis B Reactivation
It is essential to screen for HBV before starting treatment. You may want to consider taking measures as a precaution.
Tuberculosis and Other Infections
Before initiating treatment, it is important to screen patients for any underlying infections, such as tuberculosis.
IX. Drug Interactions and Compatibility
Pharmacological Interactions: Chemotherapeutic Agents, Immunosuppressants
Understanding the intricate pharmacological interactions of Rituximab, especially when combined with chemotherapy drugs and immunosuppressants, requires careful medical caution. It is essential to monitor its use to avoid potential increased cytotoxic effects or worsening of immune suppression. There is a possibility of enhanced effects when used alongside chemotherapy, and it may also have a synergistic immunosuppressive effect when taken with corticosteroids.

Food and Supplement Interactions
Although there is no evidence of harmful interactions with food, it is recommended to exercise caution when including dietary supplements with immunomodulatory properties, like echinacea or astragalus.
Compatibility with Other Intravenous Solutions
When combining Rituximab with solutions given intravenously, there are potential risks involved. These risks include the aggregation of proteins or unfavorable interactions from a perspective. It is crucial to follow compatibility guidelines strictly to ensure safety.
X. Administration to Special Populations
Administration to the Elderly: Adjusted Dosage and Special Monitoring
Elderly patients can often have a situation regarding how their bodies handle medications. This is because their kidneys may not clear drugs efficiently, and they might take multiple medications simultaneously. As a result, doctors may need to create dosage plans and closely monitor them.
Administration to Pregnant Women and Nursing Mothers: Risks and Recommendations
During pregnancy, the use of Rituximab is a decision. It should only be considered if the potential benefits outweigh the risks to the developing embryo. Mothers who are breastfeeding should also be cautious.
Administration to Children: Safety Profile and Pediatric Dosing
There is information available regarding safety in children. Dosing regimens are tailored for individuals based on factors like body weight and surface area.
XI. Overdose Management and Handling Precautions
Symptoms of Overdose
While there is empirical data available, it is plausible that taking an excessive amount of Rituximab could potentially worsen known adverse effects, such as cardiovascular disturbances or the aggravation of reactions, during infusion.
Treatment and Management
To effectively manage an overdose, it is crucial to stop the infusion and provide supportive care. Ongoing medical supervision is essential in this process.
Storage Precautions: Temperature and Light Sensitivity
Ensure that the refrigeration is set at a temperature range of 2 to 8 degrees Celsius. Take care to prevent direct exposure to ultraviolet light.
Disposal Guidelines
Properly disposing of medication that is no longer needed or has expired requires adherence, to both local and national environmental regulations, with the ideal approach being the utilization of pharmaceutical take-back programs.
XII. Conclusion
Summary of Rituximab's Efficacy and Safety Profile
Rituximab has established itself as a treatment option in modern pharmacotherapy due to its versatile therapeutic uses and a favorable balance between effectiveness and potential risks.
Importance of Following Prescribing Guidelines
It is highly important. Strongly advised to strictly follow the established guidelines for prescribing medication. By doing, we can effectively reduce potential risks and improve the effectiveness of treatment.
Future Directions in Rituximab Research and Use
As scientists delve deeper into studying Rituximab they are uncovering its therapeutic possibilities. These discoveries could lead to breakthroughs in previously unexplored areas of treatment.