Flebogamma IVIG
- Introduction to Flebogamma IVIG
- Composition of Flebogamma IVIG
- Mechanism of Action: How Flebogamma IVIG Works
- Approved Uses of Flebogamma IVIG
- Off-Label Uses of Flebogamma IVIG
- Dosage and Administration Guidelines
- Flebogamma side effects
- Warnings and Precautions
- Contraindications
- Interactions with Other Medications
- Storage and Handling of Flebogamma IVIG
- Administration to Special Populations
- Overdose Management
- Important Handling Precautions
Introduction to Flebogamma IVIG
What is Flebogamma IVIG?
FleboGamm IVIG is a grade intravenous immunoglobulin solution that is utilized for addressing a range of immunodeficiency and autoimmune disorders in individuals experiencing compromised or dysfunctional systems.The therapy plays a role, in supplying antibodies to patients and is well regarded for its effectiveness standing as an integral component, in the realm of immunotherapy treatments.
Overview of Intravenous Immunoglobulin (IVIG) Therapy
Intravenous immunoglobulin (IVIG) therapy is a clinical intervention that delivers antibodies directly into the bloodstream. These antibodies, derived from plasma, enhance immune system functionality. IVIG therapy is pivotal in managing primary and secondary immunodeficiency disorders and certain autoimmune diseases.
Manufacturer and Approval Status
Flebogamma IVIG is manufactured by Grifols, a globally recognized biopharmaceutical company. The product has received approval from regulatory bodies such as the FDA and EMA, ensuring its safety and efficacy in treating eligible patients.
Indications for Use
- Treatment of Primary Immunodeficiency (PID)
- Management of Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
- Supportive care in Idiopathic Thrombocytopenic Purpura (ITP)
- Therapeutic intervention in Kawasaki Disease
Composition of Flebogamma IVIG
Active Ingredients
The main effective ingredient, in Flebonorma IVIG is immunoglobulin G (IG) an antibody protein that strengthens the bodys defenses, with IG making up 95 percent of the solutions content.
Additional Components and Stabilizers
Flebobamma IVIG includes quantities of stabilizers, like glycine, from IgE to uphold the solution's consistency and hinder protein clumping while stored.
Variants and Concentration Options
Fleborgamma IVIG comes in 5 percent and 10 percent strengths, designed to address specific treatment requirements and patient preferences.
Mechanism of Action: How Flebogamma IVIG Works
Immune System Modulation
Fleebogamma IVIG helps regulate the system by countering self-produced antibodies and restoring balance to the immune system—and it has an especially helpful impact on conditions related to the body attacking itself.
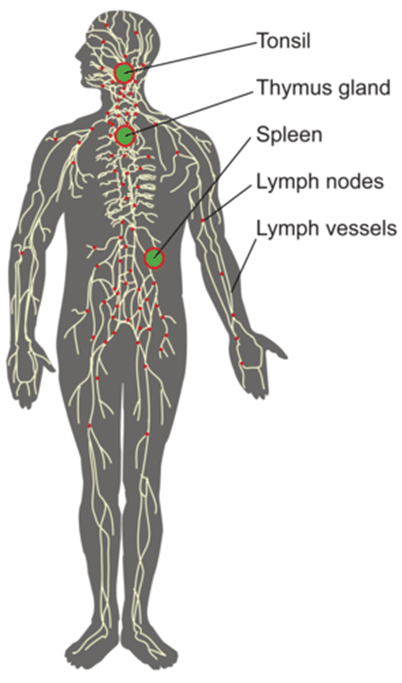
Anti-inflammatory Effects
The solution exerts anti-inflammatory properties by inhibiting the activity of pro-inflammatory cytokines and complement pathways. This reduces inflammation and tissue damage.
Role in Immune Deficiency and Autoimmune Disorders
Flebogamma IVIG protects patients with immune deficiencies from infections by supplementing deficient antibodies. Additionally, it helps regulate the overactive immune responses seen in autoimmune conditions.
Approved Uses of Flebogamma IVIG
Primary Immunodeficiency (PID)
Chronic Inflammatory Demyelinating Polyneuropathy (CIDP)
Idiopathic Thrombocytopenic Purpura (ITP)
Kawasaki Disease
Off-Label Uses of Flebogamma IVIG
Guillain-Barré Syndrome
Multiple Sclerosis (MS)
Myasthenia Gravis
Neuromyelitis Optica
Systemic Lupus Erythematosus (SLE)
Dosage and Administration Guidelines
Standard Dosage Recommendations
Dosage amounts differ depending on the ailment being addressed; for inflammatory disease (PID), a common dosage ranges from 300 to 600 mg per kilogram of body weight every 3 to 5 weeks.
Administration Techniques and Equipment
- Administered intravenously through an infusion pump
- Initial infusion rate starts slow to monitor for adverse reactions
Monitoring During Administration
Patients require close monitoring for signs of allergic reactions, including rash, hypotension, and dyspnea.
Adjustments for Specific Patient Groups
Dosage adjustments may be necessary for pediatric, geriatric, and renally impaired patients to minimize side effects.
Flebogamma side effects
Common Side Effects
- Headache
- Nausea and vomiting
- Fatigue and fever
Serious Side Effects
Warnings and Precautions
General Safety Considerations
Make sure that the patients stay well hydrated to lower the chances of kidney issues, and be careful with people who have a history of blood clotting problems.

Risk of Transmissible Agents
Although thorough screening helps lower the chances of harm plasma based goods have a chance of passing on infections.
Importance of Hydration Before Administration
Staying properly hydrated is important to reduce the chances of blood clotting issues and kidney problems when undergoing treatment.
Special Populations: Risk Factors
Specialized care is essential for women, seniors, and individuals with health conditions to reduce potential risks effectively.
Contraindications
Known Hypersensitivity to Immunoglobulins
Flebogamma IVIG should not be used in individuals who have shown hypersensitivity to immunoglobulin proteins in the past, as it can result in reactions, like anaphylaxis, with potentially life-threatening consequences. To ensure safety and avoid any reactions to immunoglobulin therapy, the prior patient history must be carefully reviewed.
Patients with Selective IgA Deficiency
People who have Igo deficiency and antibodies against IgA at risk of experiencing anaphylactic reactions when given Flebogama IVIG treatment. It is crucial to assess this condition carefully and consider other treatment options that may be more suitable.
Severe Renal Impairment
Renal issues are a known concern when it comes to receiving treatment. Acute kidney problems are something to watch out for. It is not recommended that someone already has kidney problems before starting therapy because they are more likely to experience kidney toxicity. In cases of kidney conditions, though, adjustments or different formulations of the treatment may be needed.
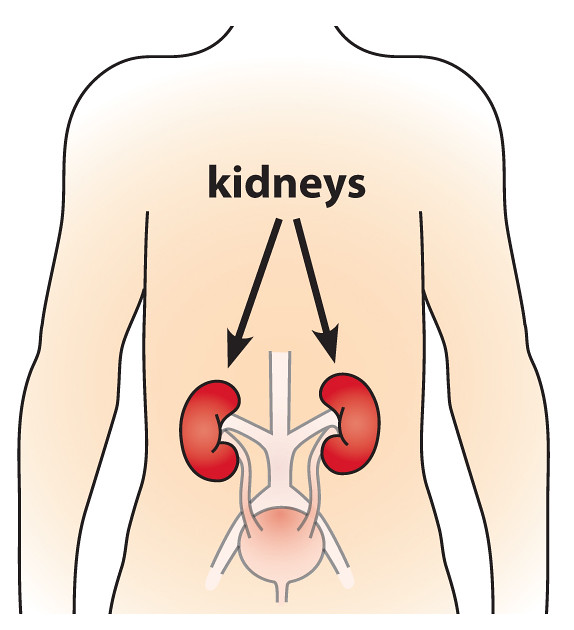
Interactions with Other Medications
Vaccines and Immunoglobulins
Flebo gamma IVIG might affect how live attenuated vaccines, like measles, mumps, and rubella (MMRs), work. To ensure the vaccines work properly, it's best to delay getting vaccinated for around three months after receiving IVIG. Patients need to be informed about when to get their vaccines to ensure they are fully protected.
Anticoagulants and Anti-platelet Drugs
Concurrent use of anticoagulants or anti-platelet agents may amplify the risk of thromboembolic events during Flebogamma IVIG therapy. Regular monitoring of coagulation parameters and signs of thrombosis is advisable when these medications are co-administered.
Other Immunosuppressive Therapies
Immunosuppressive agents can potentiate the immunomodulatory effects of Flebogamma IVIG, increasing the risk of infections. Careful assessment of the patien's immune status and dose adjustment may be warranted to minimize adverse outcomes.
Storage and Handling of Flebogamma IVIG
Recommended Storage Conditions
Store Flebogamma IVIG at temperatures between 2°C and 8°C, avoiding freezing to prevent denaturation of proteins. Protect the product from direct sunlight and excessive heat to maintain its integrity.
Shelf Life and Stability
The solution maintains stability for the duration specified on the packaging when stored under appropriate conditions. Once opened, it must be used immediately or discarded to prevent contamination and loss of efficacy.
Guidelines for Reconstitution and Use
Perform the reconstitution in an environment to maintain sterility standards and avoid shaking vigorously when preparing to prevent excessive foaming and to keep the protein stable.The best practice is to administer the solution after preparation to guarantee its effectiveness.
Administration to Special Populations
Administration to Elderly Patients
Older individuals tend to be at risk of experiencing kidney and heart-related issues when undergoing treatment for a condition. It is recommended to start the treatment with the appropriate amount and keep a close eye on kidney function, hydration levels, and how well the patient responds to the treatment during the entire therapy period.
Administration to Pregnant Women and Nursing Mothers
FleboGama IVIG falls under Category C medication classification. Recommendation is to consult with a healthcare provider regarding the use of this therapy, in mothers to evaluate effects on breastfed infants considering limited available data and weighing risks, against benefits.
Administration to Children
In hospitals and clinics, the dosage of Flebagamma IVIG is adjusted according to the children's weight and health condition to ensure safety and effectiveness. Monitoring for any reactions is crucial, even though children generally respond well to Flebagamma treatments.
Overdose Management
Symptoms of Overdose
Overdose may manifest as symptoms such as headache, fever, nausea, and renal dysfunction. Severe cases could lead to fluid overload or thromboembolic events requiring immediate intervention.
Immediate Actions to Take
In case of suspected overdose, halt the infusion and provide symptomatic treatment. Monitoring renal function and vital signs is paramount. Administer supportive care to address any complications promptly.
Long-term Implications of Overdose
Sustained excessive intake could result in lasting harm to the kidneys or problems with blood vessels, requiring monitoring for lingering issues and ensuring a recovery over time.
Important Handling Precautions
Sterility and Aseptic Techniques
Maintain strict aseptic protocols during preparation and administration to prevent contamination. Use sterile syringes, needles, and infusion equipment to ensure safety.
Disposal of Unused Product
Discard any unused product following local guidelines for medical waste. Do not reuse partially used vials to avoid contamination and compromised sterility.
Avoiding Contamination During Preparation
Handle vials and equipment with care to prevent microbial contamination. Minimize exposure to air and use within the recommended time frame after opening.
Flebogamma IVIG FAQ
- What is flebogamma used for?
- Why give IVIG after plasmapheresis?
- What are the side effects of the flebogamma infusion?
- What is the drug IVIG used for?
- What is the rate of infusion for Flebogamma?
- What are the positive effects of IVIG?
- What happens when IVIG is stopped?
- How will I know if IVIG is working?
- How long does IVIG take to reduce inflammation?
- How does Flebogamma work?
- Can IVIG cause liver damage?
- Is IVIG a high risk medication?
What is flebogamma used for?
Flebotella DIF is administered to individuals requiring antibodies in their bloodstream to combat infections and various ailments.
Why give IVIG after plasmapheresis?
Through the use of treatment, the antibodies targeting the transplant can be weakened.
What are the side effects of the flebogamma infusion?
You might experience symptoms such as flushing, headaches, feeling chills, muscle cramps, back or joint pain, fever, nausea, or vomiting.
What is the drug IVIG used for?
Intravenous immunoglobulin (IVIG), a pooled antibody treatment derived from sources, is utilized to treat various immune deficiencies and a wide range of conditions, such as autoimmune diseases and inflammatory infections.
What is the rate of infusion for Flebogamma?
When your infusion starts off with Flebogamma DIF slowly (at a rate of 01 to 02 milliliters per kilogram per minute), your doctor might adjust the infusion speed based on how you're feeling and gradually increase it (up to 01 milliliters per kilogram, per minute).
What are the positive effects of IVIG?
Assisting your system by boosting the production of antibodies to enhance its natural defense mechanisms.
What happens when IVIG is stopped?
Seventy-three percent of the participants exhibited decline based on the adjusted INCAT score, with the rest showing deterioration through either I-RODS or grip strength assessments.
How will I know if IVIG is working?
You might observe a decrease in how severe your infections are.
How long does IVIG take to reduce inflammation?
After your initial IVIG treatment session is completed, it might take a few days or weeks before you start to see any changes in how you feel.If this treatment is effective for you, you should begin to experience some improvements within a span of around four weeks.
How does Flebogamma work?
Bringing the IgGs in the blood back to their usual levels.
Can IVIG cause liver damage?
An uncommon adverse reaction associated with the administration of IVIG is liver damage.
Is IVIG a high risk medication?
IVIG can occasionally result in blood pressure levels that may escalate the likelihood of experiencing a heart attack or stroke.