Diphtheria Antitoxin, Vial
- Introduction
- Composition and Formulation
- Uses and Indications
- Off-Label Uses
- Mechanism of Action
- Dosage and Administration
- Storage and Handling Precautions
- Warnings and Precautions
- Common Side Effects
- Serious Side Effects and Adverse Reactions
- Drug Interactions
- Administration Considerations in Special Populations
- Overdosage and Management
- Handling Precautions and Safety Measures
- Conclusion
Introduction
Overview of Diphtheria Antitoxin
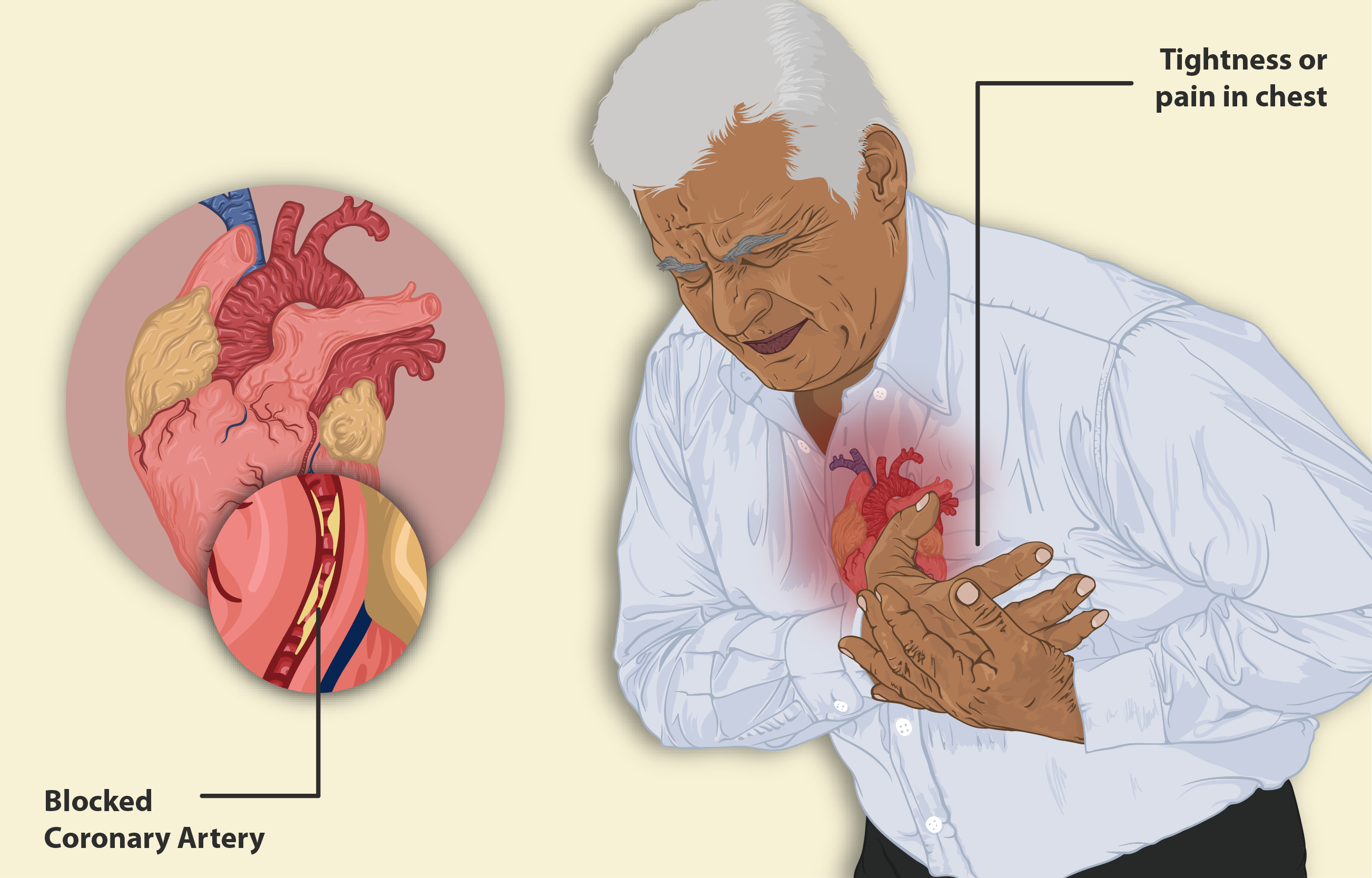
A treatment called Diphtheria Antitoxin (DAT) is a therapy made from horse plasma that is used to counteract the toxins created by Corynebacterium diphtheriae bacteria in the body to stop the disease from worsening by latching onto toxins in the bloodstream before they cause harm to tissues.
Importance in Diphtheria Treatment
Diphtheria is a potentially fatal bacterial infection affecting the respiratory tract. The primary mode of management involves DAT administration, complemented by antibiotic therapy. Timely intervention can:
- Prevent systemic toxicity.
- Reduce morbidity and mortality rates.
- Improve patient outcomes through early neutralization of diphtheria toxin.
Historical Significance and Development
Since the 1800s, when diphtheria antitoxin was first introduced for public health purposes, it has been crucial in shaping modern passive immunotherapy thanks to the groundbreaking work of Emil von Behring. Throughout the years, improvements in purification methods have helped create more effective formulations.
Regulatory Status and Approval
The World Health Organization (WHO) categorizes Diphtheria Antitoxin as a medication that undergoes quality control during production by regulatory bodies like the U.S. FDA and EMA. Antitoxin distribution is limited in some areas and mostly reserved for managing outbreaks and specific healthcare settings.
Composition and Formulation
Active Ingredients and Their Role
The main element of diphtheria antitoxin consists of antibodies collected from horses that have been hyperimmunized for this purpose. These antibodies deactivate the diphtheria toxin to stop it from entering cells and causing harm afterward.

Inactive Ingredients and Excipients
To enhance stability and patient safety, DAT formulations may contain:
- Preservatives such as phenol.
- Stabilizing agents like glycine.
- Buffering compounds to maintain pH balance.
Available Strengths and Concentrations
Different strengths of diphtheria antitoxin are accessible in amounts per vial measured in units (IU). These may come in 10k IU 20k IU or 40k IU to match the seriousness of the infection.
Pharmaceutical Form and Packaging
Manufactured as a sterile solution, DAT is supplied in single-dose glass vials. Proper packaging ensures stability during storage and transport, minimizing degradation risks.
Uses and Indications
Primary Use in Treating Diphtheria Infection
Prevention of Diphtheria Progression
Post-Exposure Prophylaxis in High-Risk Cases
During outbreaks of illnesses like diphtheria, people who have been in contact with it might get DAT as a step for those more likely to develop severe symptoms, like those who haven't been vaccinated or those with weakened immune systems.
Role in Reducing Diphtheria-Related Complications
Without intervention, diphtheria may lead to severe complications:
- Myocarditis (heart inflammation).
- Neurotoxicity affecting cranial and peripheral nerves.
- Multi-organ failure due to systemic toxin dissemination.
Diphtheria antitoxin significantly lowers these risks when administered in a timely manner.
Off-Label Uses
Experimental Use in Other Bacterial Infections
Exploratory research delves into the effectiveness of DAT in counteracting exotoxins created by pathogens, yet concrete clinical proof is still scarce.
Potential Role in Neutralizing Other Toxins
Investigational Use in Antitoxin Therapy
Studies evaluating the wider uses of polyclonal antitoxins show promise for new treatment options beyond just diphtheria therapy.
Mechanism of Action
How Diphtheria Antitoxin Neutralizes Toxins
The diphtheria antitoxin works by attaching to the diphtheria exotoxin before it connects to host cells and stops the toxin from disrupting the protein production process in cells and preventing cell death in the end.
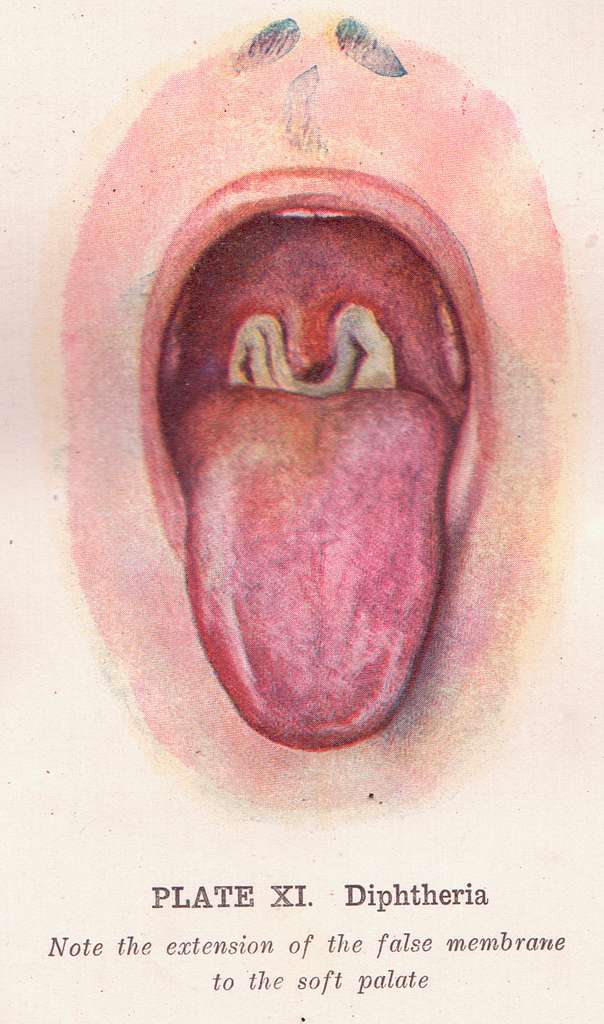
Interaction with the Immune System
DAT functions as a type of immunotherapy that delivers toxin-neutralizing antibodies without triggering the patient's adaptive immune response into action – a departure from vaccines that trigger long-lasting immunity.
Time Frame for Effectiveness
Treatment is most efficient when given soon after the disease starts to show symptoms –within 48 hours of onset. Administered late may not be able to stop existing tissue damage. Can still halt any harmful toxin effects from occurring.
Dosage and Administration
Recommended Dosage Based on Disease Severity
The dose varies depending on clinical severity:
- Mild cases: 20,000 IU.
- Moderate to severe cases: 40,000-80,000 IU.
- Systemic complications: Up to 100,000 IU.
Dosage Guidelines for Pediatric Patients
Children are given doses tailored to their weight to make sure the toxins are neutralized effectively while reducing the chances of hypersensitivity reactions.
Dosage Adjustments for Special Populations
Patients who have issues with their kidneys or liver might need adjustments in their dosage to lower the risk of side effects.
Administration Route and Techniques
The best way for absorption throughout the body is through infusion, while intramuscular administration is typically used for less severe situations or when logistical challenges arise.
Duration of Treatment and Monitoring Requirements
After giving the medication to patients, it's important to keep an eye on them for any signs of reactions or anaphylaxis.
Storage and Handling Precautions
Optimal Storage Temperature and Conditions
Store DAT at a temperature range of 2 ̊ ̊ C and keep it shielded from exposure to maintain its integrity; avoid freezing as it can harm the protein structure.
Stability and Shelf Life Considerations
In circumstances DAT remains stable for three years. Expired vials should be disposed of because they lose effectiveness over time.
Proper Disposal and Waste Management
Make sure to dispose of any vials that have not been used and syringes that have been used according to the biohazard rules to avoid any risks of contamination.

Warnings and Precautions
Risk of Severe Allergic Reactions
As a product made from horses (equine derived) DAT comes with the possibility of causing hypersensitivity reactions that may require allergy testing before administering it.
Immune System Responses and Serum Sickness
Days after receiving the medication injection, some individuals might experience symptoms of serum sickness such as fever and joint pain along with a rash, which may be attributed to the formation of complexes.
Precautions in Patients with Preexisting Conditions
Individuals with asthma or autoimmune conditions should be approached with care since adjusting the response could potentially worsen existing health issues.
Risk of Anaphylaxis and Emergency Management
Immediate treatment for anaphylaxis includes administering epinephrine and providing supportive care as necessary.
Pre-Treatment Allergy Testing Recommendations
It is recommended that skin sensitivity tests be conducted before giving the dose to prevent allergic reactions.
Common Side Effects
Overview of Mild to Moderate Side Effects
Diphtheria Antitoxin, derived from equine serum, can elicit a spectrum of side effects. Most adverse reactions fall within the mild to moderate category, resolving spontaneously without intervention. These effects generally manifest shortly after administration and may include:
- Localized discomfort at the injection site.
- Transient fever, often low-grade.
- Mild allergic responses such as itching or rash.
While not life-threatening, these reactions warrant observation, particularly in patients with heightened immune sensitivities.
Skin Reactions and Hypersensitivity Symptoms
Skin-related manifestations are among the most common side effects associated with diphtheria antitoxin. Patients may experience:
- Erythema (redness) and pruritus (itching) at the injection site.
- Urticaria (hives) ranging from localized to widespread.
- Angioedema, although rare, presents as facial or limb swelling.
Most hypersensitivity reactions are self-limiting, but persistent or escalating symptoms necessitate medical assessment.
Temporary Fever and Flu-Like Symptoms
Post-administration fever is a common physiological response, often accompanied by flu-like symptoms:
- Fatigue and muscle aches.
- Chills and mild headaches.
- Generalized malaise.
This transient reaction typically subsides within 24 to 48 hours and is manageable with symptomatic treatment.
Serious Side Effects and Adverse Reactions
Anaphylaxis and Severe Allergic Reactions
Though infrequent, anaphylaxis is the most critical hypersensitivity reaction. Symptoms develop rapidly and require immediate intervention. Signs include:
- Respiratory distress (wheezing, stridor, bronchospasm).
- Severe hypotension leading to circulatory collapse.
- Loss of consciousness in extreme cases.
Emergency measures include epinephrine administration, airway management, and supportive care.
Serum Sickness and Immune Complex Disease
Serum sickness, a delayed hypersensitivity reaction, can arise days to weeks post-treatment. Symptoms include:
- Fever, joint pain, and lymphadenopathy.
- A rash resembling vasculitis.
- Systemic inflammation affecting multiple organ systems.
Managing serum sickness involves corticosteroids and antihistamines.
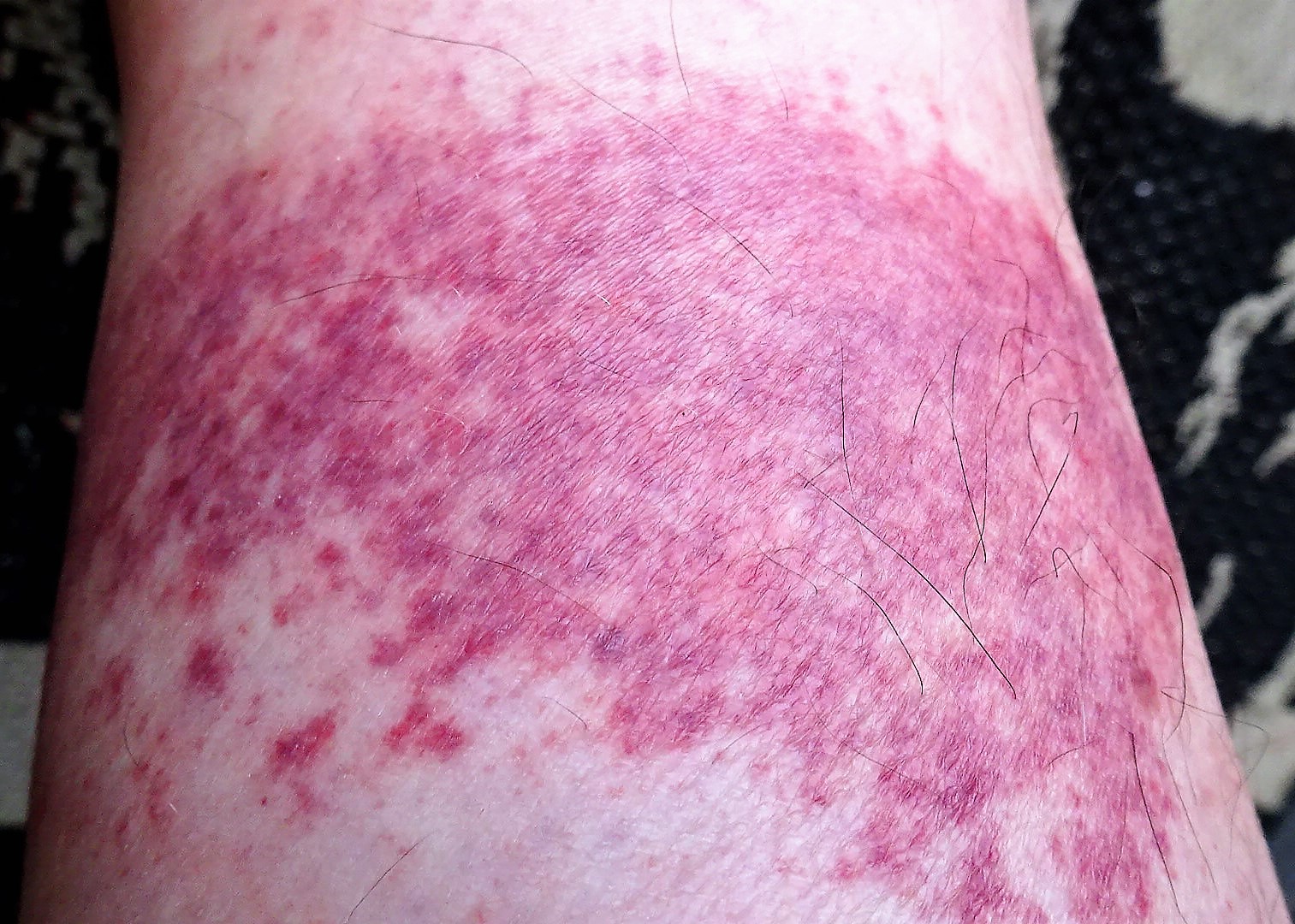
Neurological Effects and Systemic Reactions
Although uncommon, neurological side effects have been reported. These may include:
- Paresthesia (tingling or numbness in extremities).
- Transient confusion or dizziness.
- Rare cases of Guillain-Barre syndrome-like symptoms.
Long-Term Health Risks and Monitoring
The lasting impacts of antitoxins derived from horses are still being studied. Individuals who have experienced reactions, in the past should be closely monitored for delayed immune responses, after treatment.
Drug Interactions
Interaction with Other Vaccines and Immunoglobulins
The diphtheria antitoxin might affect the effectiveness of vaccines. Requires careful timing when administering them together to avoid any interference with each other’s efficacy, as well as taking into consideration how immunoglobulin therapies may also influence their performance.
Possible Interference with Antibiotics
While antibiotics complement diphtheria antitoxin by eradicating bacterial infection, interactions have been noted with:
- Penicillins and macrolides affect absorption rates.
- Tetracyclines, altering pharmacokinetics.
Drug Combinations That May Alter Effectiveness
The combined intake of corticosteroids could weaken the body's reaction to diphtheria antitoxin treatment, while therapies that suppress the system, like chemotherapy drugs, might also decrease its effectiveness.
Guidelines for Safe Co-Administration
To mitigate interaction risks:
- Space out vaccine administration by at least four weeks.
- Monitor patients receiving immunosuppressants closely.
- Consult pharmacokinetic data when co-administering antibiotics.
Administration Considerations in Special Populations
Use in Elderly Patients
Age-Related Sensitivity to Treatment
Elderly individuals frequently show increased sensitivity to treatments with worries such as a higher risk of serum sickness and slower metabolism clearance.
Dosage Adjustments and Monitoring Needs
Changes, in the body as we age mean that dosages may need adjusting and require monitoring after taking medication is crucial.
Administration During Pregnancy and Lactation
Safety Profile for Pregnant Women
There is no information about the use of diphtheria antitoxin in pregnancy; nevertheless, the transfer of passive immunity to the fetus might provide protective advantages.
Potential Risks to the Fetus
There is uncertainty about risks to the fetus at this time. If a mother experiences an anaphylactic reaction it could result in decreased oxygen supply to the unborn baby.
Use During Breastfeeding and Neonatal Considerations
There is information indicating that there is a transfer of horse-derived antibodies through breast milk.
Pediatric Administration
Safety and Effectiveness in Children
Generally speaking, children tend to handle diphtheria antitoxin, although there are more instances of hypersensitivity reactions in this age group.
Adjusted Dosage for Pediatric Patients
Optimizing dosage based on weight helps achieve the treatment results while also reducing side effects.
Overdosage and Management
Signs and Symptoms of Overdose
Overuse of medication can worsen reactions and overall inflammatory responses in the body.
Emergency Management and Medical Interventions
Treatment involves stopping the medication and using antihistamines and corticosteroids, as well as providing symptomatic relief measures.
Long-Term Consequences of Overdosage
Extended activation of the system after an overdose could lead to symptoms resembling conditions.
Handling Precautions and Safety Measures
Protective Measures for Healthcare Professionals
Using gloves correctly and maintaining a technique helps reduce the chances of contamination problems.
Proper Handling to Avoid Contamination
It's important to check the integrity of the vial before using it. If it's contaminated or expired, it should be thrown away.
Safe Disposal Practices
Proper disposal methods should follow biohazard protocols to avoid contaminating the environment.
Conclusion
Summary of Key Information
Antitoxin is still crucial for neutralizing toxins in diphtheria cases. Providing treatment support.
Importance of Medical Supervision
To prevent reactions from occurring during administration of the substance, it is important to have medical supervision present.
Future Research and Developments in Antitoxin Therapy
In the future, evolving monoclonal antibody treatments could offer antitoxin solutions sourced from humans.
Diphtheria Antitoxin, Vial FAQ
- What antitoxin is used for diphtheria?
- Why is antitoxin for diphtheria no longer used?
- Is diphtheria antitoxin IM or IV?
- When do you give diphtheria antitoxin?
- What is the difference between diphtheria antitoxin and toxoid?
- Why is an antitoxin required to treat cases of diphtheria?
- Why was antitoxin given instead the tetanus vaccine?
- How to make diphtheria antitoxin?
- Is toxoid the same as antitoxin?
- How do you give antitoxin?
- What is diphtheria antitoxin used for?
- Can you give tetanus antitoxin and toxoid together?
- Is diphtheria antitoxin the same as vaccine?
- When was diphtheria antitoxin?
- What antitoxin is used for diphtheria risk?
- What is the difference between diphtheria antitoxin and toxoid?
- How to get diphtheria antitoxin?
What antitoxin is used for diphtheria?
For years, diphtheria antitoxin (DAT), a treatment for diphtheria, has been in use, though it is no longer manufactured in the United States.
Why is antitoxin for diphtheria no longer used?
After receiving the treatment, it's common to be extra sensitive, so it's important to have resuscitation resources in case. When it comes to preventing diphtheria, we no longer rely on diphtheria antitoxin due to the risk of hypersensitivity reactions. Those who are not vaccinated should be examined promptly. Provided with antibiotics for prevention as well as the vaccine.
Is diphtheria antitoxin IM or IV?
The intravenous (IV) method is typically the favored way to administer DAT (Diphtheria Antitoxin), in situations. The dosage of antitoxin should be diluted in 250 –500 mL of saline. Infused slowly over a period of 2 – 4 hours while closely observing for any signs of anaphylaxis. In situations through, the antitoxin can also be administered intramuscularly (IM).
When do you give diphtheria antitoxin?
Administer as soon as possible.
What is the difference between diphtheria antitoxin and toxoid?
Toxoids are toxins that have been modified to create immunity before exposure. Antitoxins are antibodies designed to treat individuals already affected by toxin poisoning.
Why is an antitoxin required to treat cases of diphtheria?
Special antitoxin, for diphtheria, works to neutralize the toxin circulating in the bloodstream.
Why was antitoxin given instead the tetanus vaccine?
The antidote stops the effects of the poison. Stops the illness from spreading in this situation without the need for a vaccine since vaccines are designed to protect against repeat exposure to the same disease-causing agent used in the vaccine.
How to make diphtheria antitoxin?
Researchers cultivate diphtheria-causing bacteria in a lab setting. Extract its toxin afterward. Then, they administer the diphtheria toxin to horses. Subsequently scientists draw blood from the horses. Isolate the serum in antitoxins.
Is toxoid the same as antitoxin?
Toxoids are inactivated bacterial toxins.
How do you give antitoxin?
Mix the antidote, with saline in a 1 to 10 ratio. Administer 0 oh uh oh two milliliters subcutaneously at the beginning, before monitoring for 30 minutes.
What is diphtheria antitoxin used for?
Antitoxin, for diphtheria, is produced using horse blood. It serves as a treatment, not a vaccine, for the illness caused by Corynebacterium diphtheria bacteria, helping to halt the progression of the disease.
Can you give tetanus antitoxin and toxoid together?
Administer Toxoid, at the time, as the antitoxin. Repeat the process after 30 days.
Is diphtheria antitoxin the same as vaccine?
The fatality rate for diphtheria ranges from 5 to 10%. It is more deadly in children compared to adults or older individuals. Treating diphtheria infection effectively in the body involves giving diphtheria antitoxin to counteract the effects of the toxin produced by the bacteria and using antibiotics to eliminate the bacteria causing the illness. The diphtheria vaccine is created from a toxoid that has been rendered harmless by deactivating its properties.
When was diphtheria antitoxin?
1890
What antitoxin is used for diphtheria risk?
To avoid getting diphtheria, people who have been around the disease can use diphtheria antitoxin as a measure or treatment option.
What is the difference between diphtheria antitoxin and toxoid?
The diphtheria vaccines use a modified form of the toxin called diphtheria toxoid to trigger the production of antibodies known as IgGs against the toxin itself from the C.diphtheriae bacteria that produces it in liquid culture media and then renders it inactive using formalin treatment.
How to get diphtheria antitoxin?
Diphtheria antitoxin is only available in hospitals. Your doctor will have to request it through an order.